I wouldn’t be surprised if you’ve heard the word “quantum” before. It’s a real buzzword: “quantum computing,” “quantum gravity,” “quantum information,” “quantum entanglement”. But what is quantum mechanics, really? My goal in this post is to give you intuition for what quantum mechanics is, where you can find it in real life, and why it’s so important.
What is quantum mechanics?
Officially, quantum mechanics is what we use to understand the physics of matter at subatomic scales. Informally, quantum mechanics is the set of tools we use to describe how things behave when they’re really, really small. You probably know what happens to a ball when you throw it across the room – it will travel in an arc from one end to the other, hit a wall, and fall to the ground and keep rolling. But how do things much smaller than that behave?
The central idea of quantum mechanics is something called the “uncertainty principle,” which is just physicists’ way of saying that when you zoom in on something tiny, your picture gets fuzzy. You lose resolution (Figure 1).
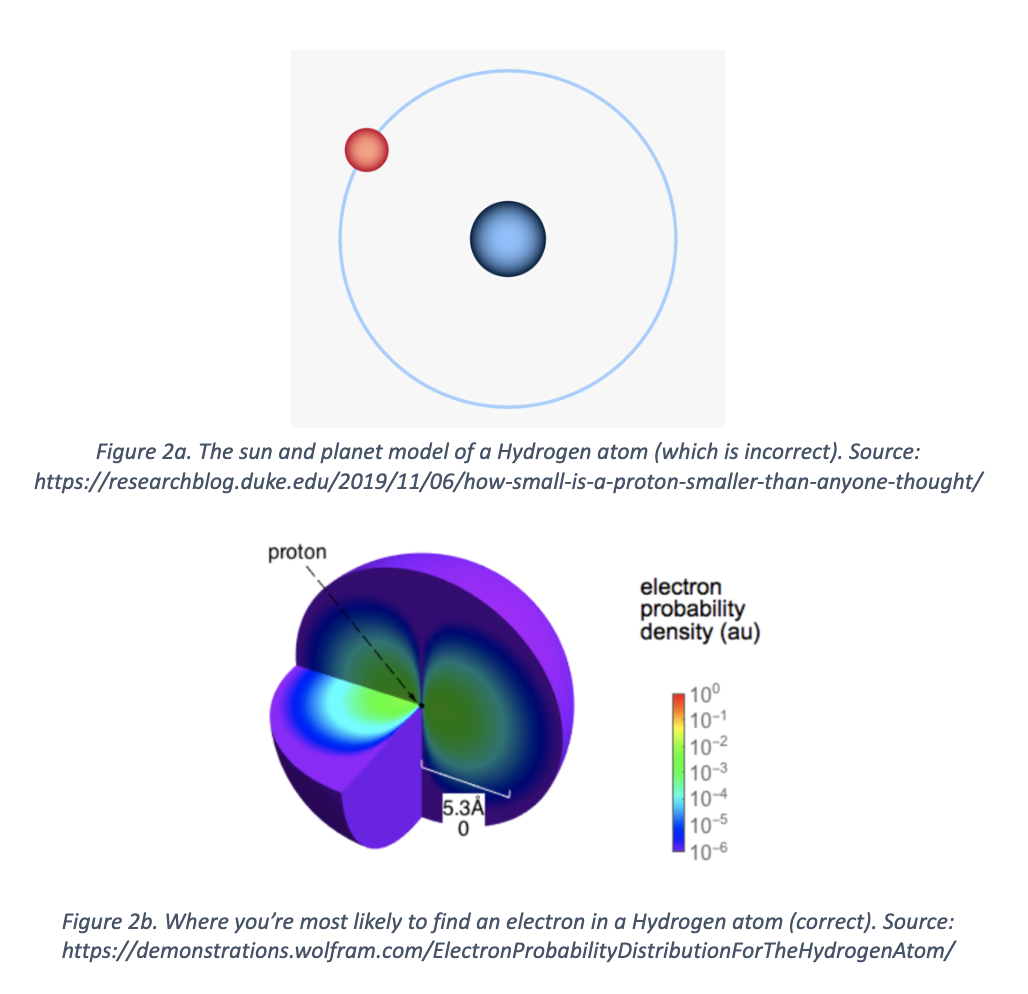
The same thing happens with subatomic (smaller than an atom) particles (things). If you try to understand where an electron is as it orbits the nucleus of a Hydrogen atom, your picture gets blurrier. You don’t know for certain. This pokes a hole in the “sun and the planets” model of atoms, which you may have seen in a chemistry class somewhere (Figure 2a, 2b).
Where can I see quantum mechanics in real life?
Let’s do an experiment. Find a sharp pencil or pen and try to balance it on the pointy end on your desk. Can you?
Probably not. The uncertainty principle tells us that we don’t know for sure where every atom in the pencil is located, which means that if we were to try to align every atom in the pencil one on top of the other by zooming in with a powerful microscope, our picture would get fuzzy. You don’t know for sure whether all the atoms of the pencil are in the kind of arrangement that would keep the pencil upright, and so inevitably, some of the atoms are misaligned, and the pencil falls over. If you do the math, you can compute that the longest you can balance a pencil on its tip is only a few seconds (many college physics textbooks will sarcastically say that this is “an exercise left to the reader”).
Why is quantum mechanics important?
A basic example of quantum mechanics in action are solar panels; quantum mechanics tells us that sometimes an electron can get excited when hit with a photon (a particle of light) and gives us a bit of energy that we can harness as electricity.
Quantum mechanics also may allow us to build far more powerful computers that we can use for, well, anything. Quantum computing is building computers that instead of using 0s and 1s to make decisions, use a “blurry” picture of 0, 1, and everything in between, unlocking much higher computational capability.
But what’s more, quantum mechanics gives us an understanding of the most basic building blocks of all matter, which in turn, sheds light on how the universe has come to be.

Comments