
In the words of The Offspring, you gotta keep 'em separated.
Last semester I was a teaching fellow for an undergraduate science class at Harvard. The class focused on both biology and chemistry, and it moved pretty quickly. Before the students knew it, we were taking about chemical reactions and going over thermodynamics. Having served as a teaching fellow for several undergraduate science classes and as an advanced chemistry tutor for several years in Boston, I was aware that students sometimes find it difficult to fully understand the information provided by thermodynamics, and they can even confuse thermodynamics with kinetics. So, let’s go over thermodynamics quickly and point out where and how it differs from kinetics.
Thermodynamics focuses on the flow of energy within a system, and it helps predict the condition under which it is favorable for a chemical reaction to occur. Chemical systems are more stable at lower energy levels; thus, going from a higher energy state to a lower energy state is preferred. During this high-to-low-energy transition, energy is released and we say that the reaction happens spontaneously. So, in a chemical reaction, by knowing the energy level (referred to as Gibbs free energy) of the reactants and the products, we can predict which reaction will happen spontaneously. The forward reaction (reactants to products) will happen spontaneously if products have lower energy than reactants, and the reverse reaction (products to reactants) will happen spontaneously if the reactants have lower energy than the products.
BE CAREFUL-saying that a reaction happens spontaneously doesn’t tell us anything about the speed with which it happens. Our other friend, kinetics, provides that information.
When we talk about chemical reactions, we usually always talk about equilibrium as well. Equilibrium is energy minimum of a system, and it is composed of both reactants and products. It is NOT composed of equal parts of reactants and products, but the rate of the forward reaction is equal to the rate of the reverse reaction at equilibrium (again, we are using kinetics here, because rate has to do with the speed of the reaction). Equilibrium is characterized by the equilibrium constant (Keq), which is determined by the concentration of the products and reactants at equilibrium. A general formula for the equilibrium constant is shown below:
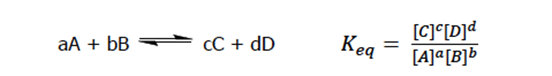
The equilibrium constant tells us whether the equilibrium favors the reactants or products. For instance, if Keq<1, there are more reactants at equilibrium, and if Keq>1, there are more products at equilibrium. If Keq=1 then the equilibrium is a 50-50 mixture of reactants and products.
Let’s consider the reaction below.

The thermodynamic information for this reaction would be summarized in the graph below:
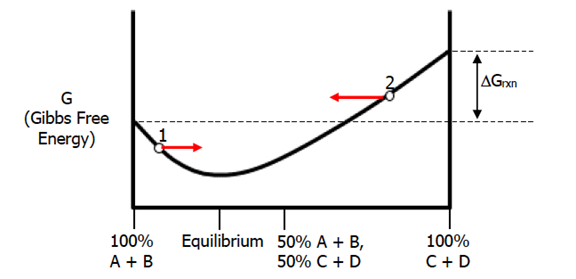
As you can clearly see from this graph, the products (100% C+D), are higher in energy that the reactants (100% A+B) which means that the reverse reaction is favored (products to reactants). Also, in this case, the equilibrium point has less products than reactants (equilibrium point is to the left of the 50% graph point). This means that Keq in this case is less than 1. Note here how the equilibrium point does not need to have equal amounts/portions of reactants and products. The equilibrium point tells us that at that particular point in the reaction, the system is at an energy minimum and that the rate of the forward and reverse reactions are equal at that point. Since chemical systems attempt to reach energy minimums, if the chemical system is at point 1 it will move right towards the equilibrium point. Using the same logic, the chemical system at point 2 will move left toward the equilibrium point.
Kinetics, on the other hand, provides us with information on how fast a certain reaction will occur. A reaction can be spontaneous, meaning it does not require additional energy to occur and results in the release of energy, but it can be very slow. The bottom line is: there is no necessary relationship between the kinetics of a reaction and thermodynamics of a reaction.


Comments