Deprotonation
An acid is a molecule that gives up a proton. Once an acid has given up the proton, it is said to be “deprotonated.” Below is the formula describing the protonated and deprotonated state of an acid:
The right side of the arrow represents the deprotonated state, whereas the left side represents the protonated state. A- is called the conjugate base of acid A-H.
But note that this equation focuses only on the acid being deprotonated, and it makes it seem like this process is happening in isolation. This is not quite true: these reactions happen in an aqueous environment, which means we the equation above is missing a very important player: water. So, let’s fix the mistake and represent the equation the way it actually happens.

Now, let’s talk a bit about the kinetics of this reaction. Based on what you might have already learned about kinetics, you can probably write an equation of the Keq for this reaction.
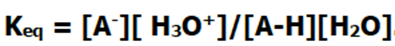
However, the concentration of the water present is far greater than that of the acid, and its concentration doesn’t change much during the course of the reaction. This means that we can actually define a new constant, which accounts for the concentration of water.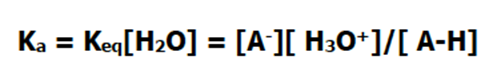
This new constant, Ka, is called the acid dissociation constant. Sound familiar? It probably does, since you have heard about this constant when talking about acids.
Don’t let this new constant rattle you: it is simply a slightly modified Keq. Rememeber: the larger the Ka of an acid is, the more likely it is that the acid will exist in the deprotonated state, and therefore be a strong acid. The smaller the Ka, the more likely it is that the acid will exist in the protonated state, and therefore be a weak acid.
However, Ka values can be very large or very small, so to make dealing with them more convenient, scientists usually use the value pKa.
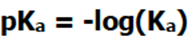
The new equation tells us that the smaller the pKa, the stronger the acid and the bigger the pKa, the weaker the acid. Also note that when the Ka changes by an order of magnitude, the pKa will only change by one unit.
However, when we talk about an environment, we rarely talk about pKa or Ka. Instead we talk about acidity. The acidity of a solution or environment refers to the concentration of protons in solution, which in turn depends on the strength of the acid present in solution. The stronger the acid, the more protons there will be in solution, and the more acidic the solution. However, the concentration of protons can very small or large too, which leads scientist to use pH instead.

Using the same reasoning as in the pKa case, the higher the concentration of protons in solution (strong acid), the lower the pH and the more acidic the solution.
Henderon-Hasselbalch equation
Be careful to keep pKa and pH separate. They certainly affect each other, but they are not the same thing. pKa is inherent to an acid and remains the same whatever the pH of the solution. pH on the other hand only describes the concentration of hydronium ions in solution. Said in another way, pH is a reflection of a solution whereas pKa is a property of a molecule. In fact, there is an equation that clearly describes the relationship between pKa and pH, and it is called the Henderon-Hasselbalch equation.

This equation, and the concepts covered here, will be very useful in helping you succeed in the advanced biology and chemistry classes you end up taking.


Comments