Today we will discuss VSEPR (pronounced “vesper”), which stands for valence-shell electron-pair repulsion. The basis of VSEPR is that the electrons in bonds and lone pairs repel each other. To minimize the instability that results from these repulsions, a molecule will adopt the shape that places electron groups as far apart as possible. VSEPR theory helps us predict the shapes of simple molecules such as CO2 or NH3, but it’s much less helpful for bigger molecules such as glucose or DNA, whose shapes are governed by many additional factors.
What simple molecules are we talking about? Any polyatomic molecule in which a central atom is surrounded by up to six electron groups (either bonded atoms or lone pairs) will work. Examples include:
- CO2 (a central carbon with two bonded oxygens)
- NH3 (a central nitrogen with three bonded hydrogens and one lone pair)
- PCl5 (a central phosphorus with five bonded chlorines)
According to VSEPR, there are five fundamental geometries that a molecule can adopt:
- Linear (a central atom with two electron groups)
- Trigonal planar (a central atom with three electron groups)
- Tetrahedral (a central atom with four electron groups)
- Trigonal bipyramidal (a central atom with five electron groups)
- Octahedral (a central atom with six electron groups)
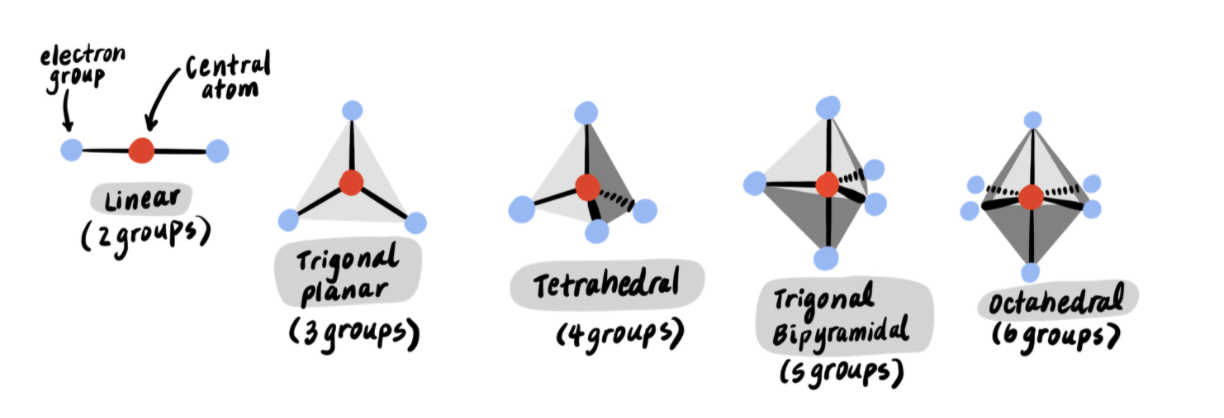
Because lone pairs are invisible, however, we must further specify the geometry that a molecule adopts. For example, NH3 has three bonds and one lone pair, for a total of four electron groups. Though the suggested geometry for four electron groups is tetrahedral, we must note that the lone pair is invisible. Therefore, the molecule actually resembles a triangular pyramid. The official name for this geometry is “trigonal pyramidal.”
The following table shows the molecular geometries that arise from all combinations of bonds and lone pairs.
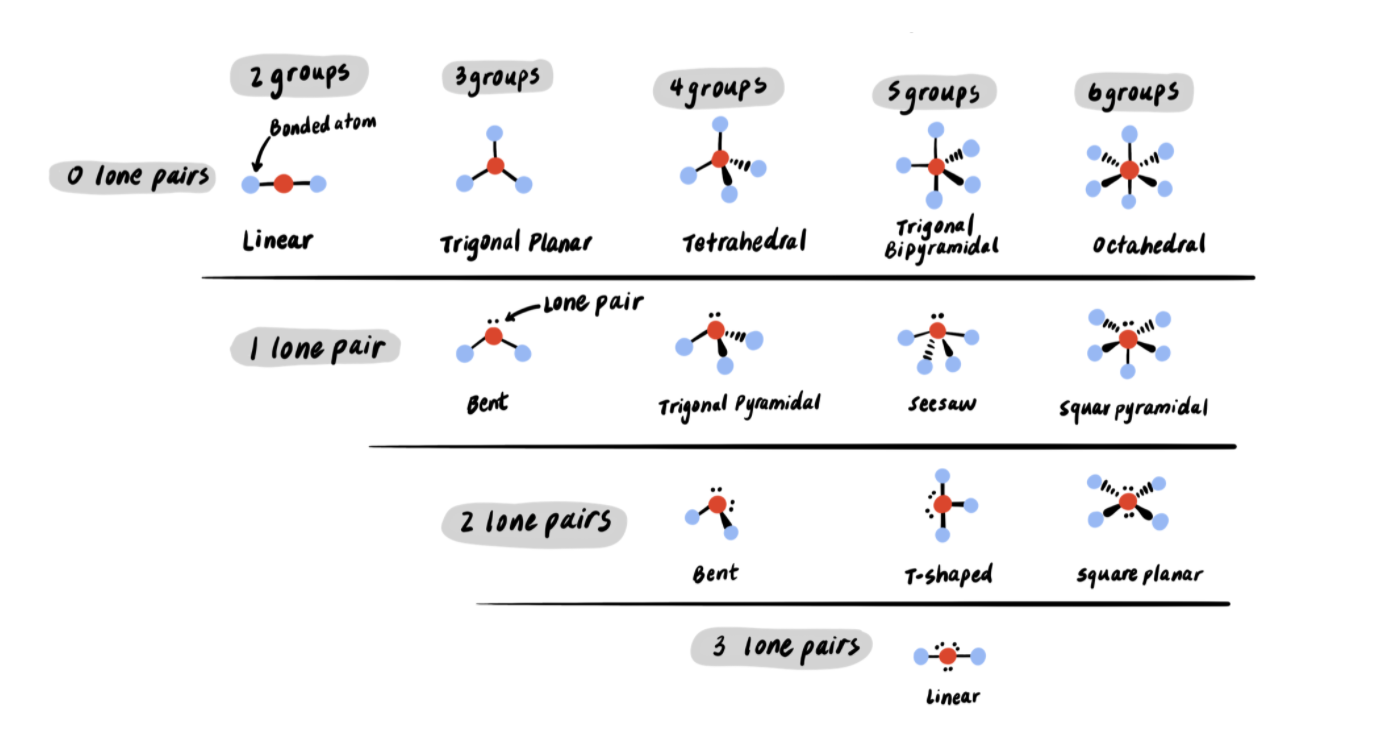
Let’s try some examples.
Example 1: Predict the molecular geometry of H2O
H2O has a central oxygen bonded to two hydrogens. It also has two lone pairs. Looking at the table, its molecular geometry is bent.
Example 2: Predict the molecular geometry of BrF5
BrF5 has a central bromine bonded to five fluorines. It also has one lone pair. Looking at the table, its molecular geometry is seesaw.
For practice, draw the table of geometries for yourself and commit the shapes to memory. It isn’t as hard as it sounds because you can predict the geometries by thinking about how best to spread the electron groups as far apart as possible. Good luck with your studying!

Comments