 Dimensional analysis is the best way to do math in chemistry. With dimensional analysis, you don’t need to memorize formulas, and you can easily check your work for every problem. Because this skill is so important, it’s crucial to have a step-by-step method that you follow every time you do it.
Dimensional analysis is the best way to do math in chemistry. With dimensional analysis, you don’t need to memorize formulas, and you can easily check your work for every problem. Because this skill is so important, it’s crucial to have a step-by-step method that you follow every time you do it.
Let’s walk through the following problem:
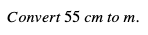
This particular problem may be one you can do in your head, but we’ll still use dimensional analysis to solve it so that you can apply the same strategy to much more difficult problems.
Step 1: Identify the unit that you’re starting with (let’s underline it), and identify the unit you want to end up with (let’s circle it).
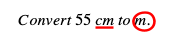
Step 2: What is the relationship between those two units? You’ve probably learned that there are 100 cm in 1 m. We can write this equality as such:
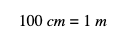
Step 3: Using the equality you just wrote, write two fractions. For our example, this would look like:
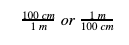
Before we decide what to do with these fractions, let’s review a bit of basic math. What would you do if someone asked you to solve this math problem?
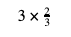
To make this clearer, you can always write a whole number (the number 3) as a fraction with the whole number (3) on the top and 1 on the bottom:
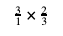
If you see the same thing on the top of one fraction and on the bottom of another fraction (and those two fractions are being multiplied), you can always cross-cancel:
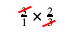
So, both 3s go away, and you’re left with 2 divided by 1, or simply 2.
With that background, let’s continue with our dimensional analysis problem.
Step 4: Write down the number you started with in the problem (55 cm). Then, the fraction you wrote in Step 3 that allows you to cancel out the unit you started with (cm), and multiply. In our example:
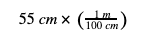
Why did we pick that fraction? Remember that 55 cm alone is the “top” of a fraction, because you could write it as 55 cm divided by 1. So, we needed to pick the fraction we wrote in Step 3 that had cm on the bottom of the fraction. This lets us cross-cancel the units:
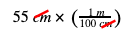
Now, that both cms are gone, and we’re left with the unit we want: m! Now finish the math, and you get your final answer of 0.55 m.
Here are some more difficult chemistry problems where this strategy can be very helpful:
You have 28 g of gold, which has a density of 19.32 g/mL. What volume of gold do you have?
All that density value means is that there is 19.32 g of gold in 1 mL of gold, so if you rewrite it as 19.32 g = 1 mL and follow the method we went through above, you can easily convert g to mL using dimensional analysis.
In 0.25 L of a 3.5 mol/L NaCl solution, how many mol of NaCl are present?
Again, rewrite the solution concentration as 3.5 mol = 1 L, and then you can simply convert units using our step-by-step method.
Now you can say goodbye to your formula sheet and use dimensional analysis to do all the math you need to do in chemistry!
Chemistry is one of our most popular subjects and we have helped countless students conquer chemistry. Our chemistry tutors are doctoral candidates and PhDs. Our team also includes MD candidates, MDs, and MD-PhDs. Most of our tutors have served as teaching assistants for hundreds of undergraduates, many of whom are encountering chemistry for the first time. Our tutors can work with students of all ages to deepen their study of chemistry at any level.
Many of our tutors specialize in teaching introductory courses, and making chemistry accessible to their students. Others specialize in preparing students for standardized tests such as the Chemistry SAT II, AP, or GRE, as well as the MCAT. We have access to the standard text books, and many of our students choose to work with a tutor in advance of, or concurrent with, challenging high school or college chemistry classes. We can easily combine chemistry tutoring with tutoring in biology, physics, and mathematics. We do our best to match students with tutors who are most appropriate to their level and learning style.
Need help with your chemistry studies? Check out some of our other helpful blog posts below!
How to begin preparing for the SAT subject test in chemistry
Formal Charge: What They Didn’t Tell You in your General Chemistry Class
Organic Chemistry: This Subject Gives You Alkynes of Trouble!

Comments