
Thanksgiving dinner conversations can be uncomfortable… but solution composition problems don’t have to be! Recall that a solution is a homogenous mixture of two or more substances. Chemists have come up with many ways to describe the composition of a solution. Some ways are more appropriate than others depending on the situation.
Here are ways of describing a solution that you’ll get very cozy with:

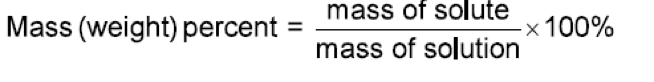


Just few equations to potentially memorize and plug some numbers into. Sounds easy. But that’s not always the case. Especially when you’re not given what you need directly. Instead you’re given a bunch of other things with the expectation that you can decipher what to do with it. In this case, it’s incredibly important to have a systematic approach to the problem or you will surely get yourself stuck.
Here are three crucial things to remember when solving a complex solution composition problem:
- EVERYTHING PROVIDED IS RELEVANT AND CONNECTED.
- YOU DID SOMETHING WRONG IF YOU DIDN’T USE EVERYTHING PROVIDED.
- FIGURE OUT HOW EACH THING PROVIDED RELATES TO WHAT YOU ARE ASKED TO FIND.
The step-by-step approach to complex solution composition problems
- Identify what the question is asking you to calculate.
- Separate that into its components. You’ll be calculating them as separate entities.
- For example, imagine that a question is asking you to calculate the molarity of a solution. Draw a line to split your work area in half. Title one half “moles of solute” and title the other half “liters of solution”.
- Place the given information under whichever title it most relates to
- This will require you to take a very close look at the given information and remember some connecting principles.
- Be thorough. Proper labeling is key. Knowing what is being measured is just as important as the unit of measurement. Hint: there’s a huge difference between the words solute, solvent, and solution.
- Using the given information that relates to them, calculate the components.
- Put the components back together to generate a final answer.
The step-by-step in practice: an example
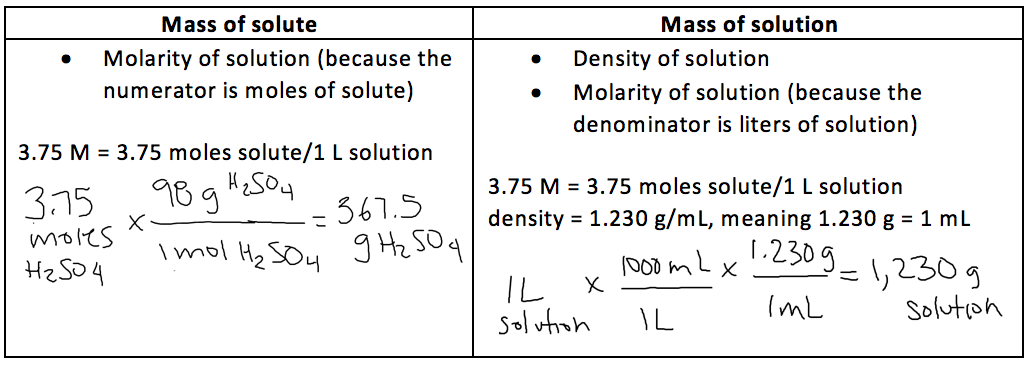
Electrolyte in automobile lead storage batteries is a 3.75 M sulfuric acid solution that has a density of 1.230 g/mL. Calculate the mass percent and molality of the sulfuric acid.
We are asked to find two different things so we will be performing the steps twice.
Let’s start with mass percent:
1. Mass percent
2. 
3. 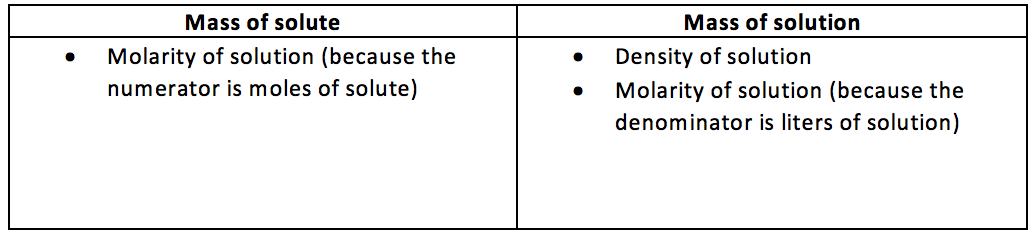 4.
4. 
5. 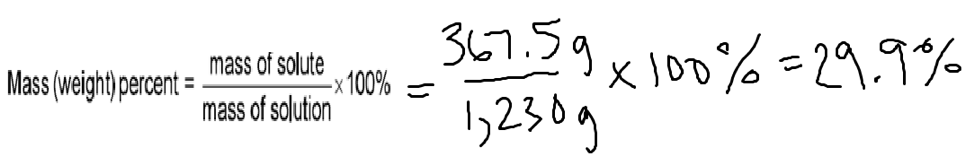
Now, let’s find the molality:
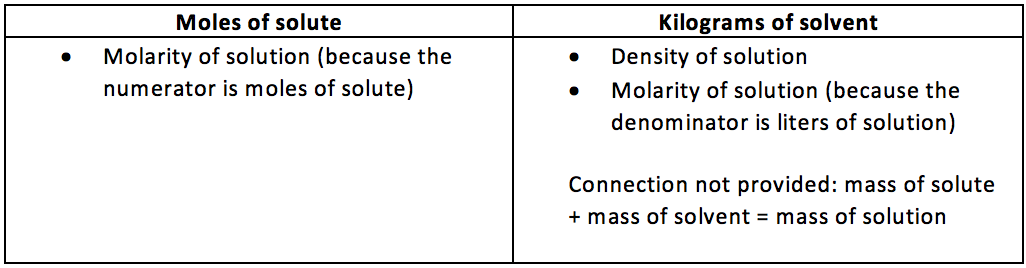
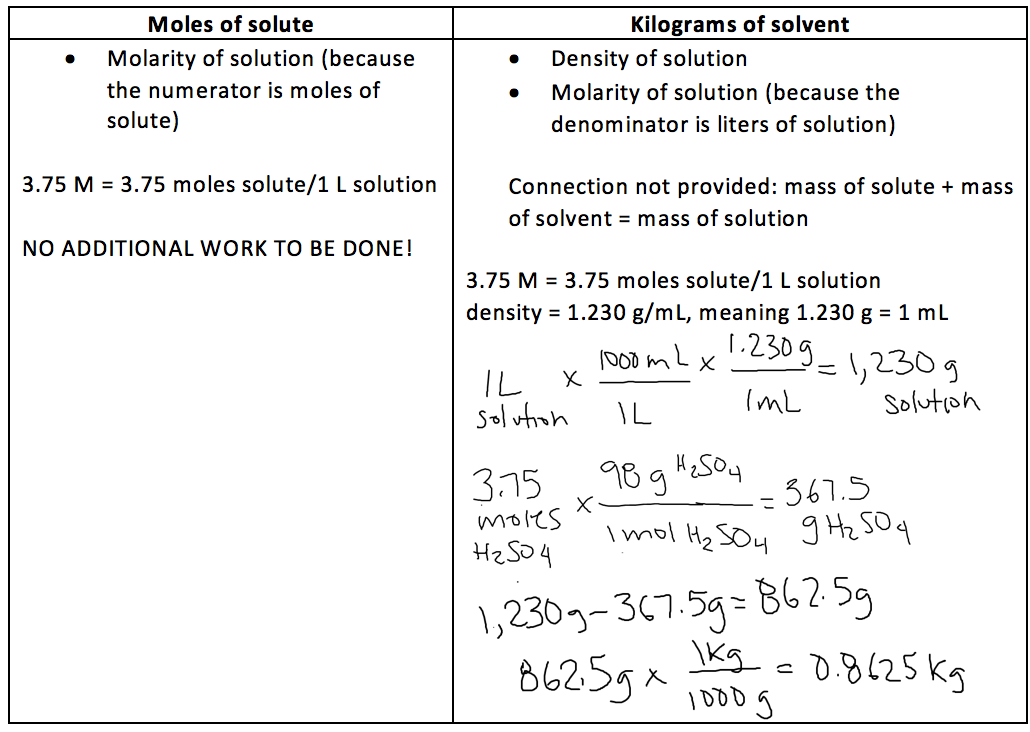
1. Molality
2. 
3. 
4. 
5. 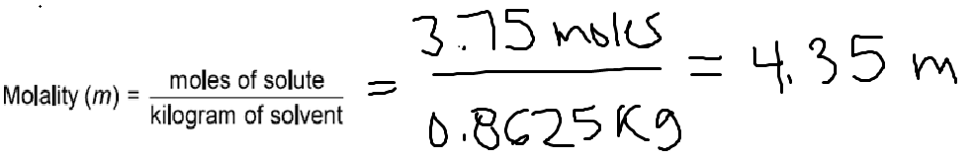
Practice truly makes perfect but keeping organized and making sure everything given in the problem is used can help hit the ground running.

Comments