If you haven't read Part 1 of this blog, please do so now.
How to Solve an 1H NMR Spectrum
1. Calculate the degrees of unsaturation (DOU).
Calculate the DOU from the molecular formula, or the number of double bonds or rings. In order to solve an NMR spectrum, a molecular formula must be provided. If not, only generalities can be determined about the structure.
Note: A phenyl ring will have a DOU=4, so check the NMR spectrum for the presence of aromatic protons (6.5 – 8 ppm region).
2. Make a list of peak positions.
Make a list including the multiplicity/splitting pattern and integration value. The number of peaks in the spectrum corresponds to the number of groups of equivalent hydrogens in the molecule.
Keep in mind that symmetry in a molecule can put hydrogens in the same chemical environment, making them appear on the spectrum as one peak.
3. Remember N+1 Rule.
Splitting pattern reveals the N+1 Rule, which states that a peak’s splitting pattern will be the number of neighboring protons (N) + 1. For example, a triplet peak indicates the hydrogen represented has 2 neighboring hydrogens.
Note: Only protons on adjacent carbons count as “neighbors.” Protons that are on neighboring heteroatoms do not count.
4. Check that the integration of the peak matches the number of hydrogens in the molecule.
If not, figure out where there are multiple identical environments in the molecule.
Hint: Remember that all the integrations of the spectrum are made with respect to one another. Therefore, if your integrations are not adding up to the total number hydrogens in your molecule, you can multiply ALL the integrals by some whole number to get the correct number of hydrogens.
5. Begin drawing.
Begin drawing possible fragments of the molecule that each peak could represent – use knowledge of integration value, splitting pattern, and chemical shift.
If you have access to the 13C spectrum, see how many peaks (equivalent carbons) are present in the molecule. For example, if the molecule has 6 carbons but the spectrum only shows 3 peaks, then you know there must be symmetry somewhere in the molecule – this will translate to your 1H spectrum, as well.
6. The rest is just a puzzle.
This is often what people love about organic chemistry. Put the fragments together until everything matches: number of hydrogens, the molecular formula, the splitting pattern, the DOU, and the chemical shift. Refer to the 13C or IR spectra if available to confirm the assignment.
NMR assignments of an unknown structure often require a lot of guess and check. But don’t be discouraged. This should be fun! With practice, assignment NMR spectra will become routine.
Let’s look at an example
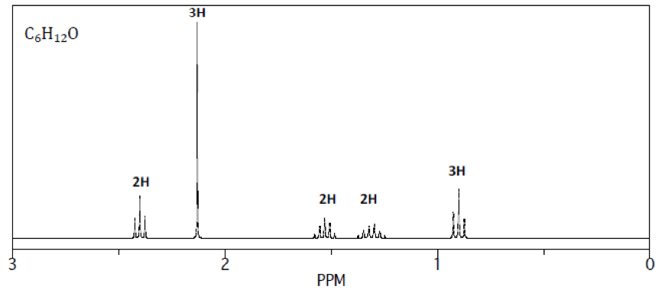
1. Let’s find the DOU for this compound C6H12
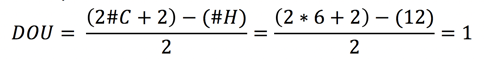
This means that our compound has either 1 double bond or 1 ring.
2. Now let’s list out the peak positions and multiplicities.
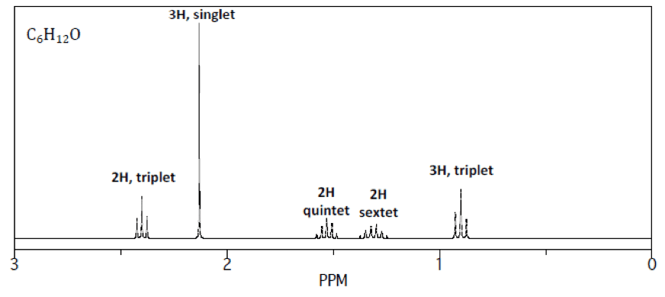
Based on the NMR spectrum, there are 5 groups of equivalent hydrogens in this molecule.
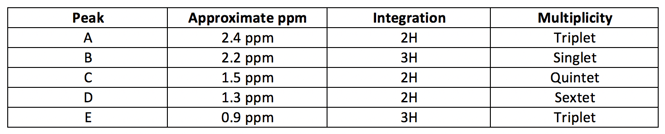
3. We have 12 hydrogens in our molecule, and the integration values in the NMR spectrum add up to 12…therefore we do not need to normalize the integrals in any way. Easy!
4. Next, look at the splitting pattern of the peaks to determine how many neighboring hydrogens are present.
Remember: Neighbors + 1 = splitting pattern.
- Triplet = this hydrogen has 2 neighboring hydrogens on adjacent carbons.
- Singlet = this hydrogen has NO neighboring hydrogens.
- Quintet = this hydrogen has 4 neighboring hydrogens on adjacent carbons. Note: since carbon can only make 4 bonds at most, these hydrogens are likely split over the two carbons on either side of the hydrogen-containing carbon in question.
- Sextet = this hydrogen has 5 neighboring hydrogens on adjacent carbons. Note: again these hydrogens are split over the two carbons on either side.
- Begin figuring out fragments.
- A group of 3H hydrogens is likely a methyl group (i.e. –CH3) – this is a pattern to remember
- A group of 2H hydrogens is likely a methylene group (i.e. –CH2–) – this is also a pattern to remember!
- The oxygen can either be a carbonyl group (C=O), an ether (C-O-C), or an alcohol (C-OH). Since there isn’t a characteristic broad singlet peak of an alcohol in the spectrum, we likely have a carbonyl or an ether.
- Solve the puzzle!
Based on what we’ve uncovered so far, this molecule has 3 methylene groups, 2 methyl groups, and a carbonyl or ether Don’t forget that we have 1 DOU. Let’s update our table.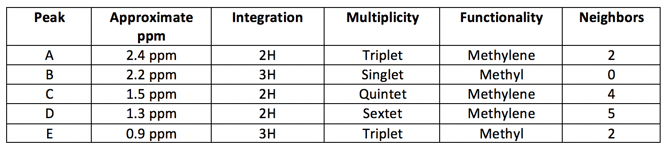
Since Peaks A and B are shifted more downfield compared to the others, these are likely close to the oxygen. B has no neighbors at all, which indicates it is right next to the oxygen. However, a hydrogen near an ether is closer to the 4 ppm range (revisit chemical shift table), so we have a carbonyl We have also found our 1 DOU!
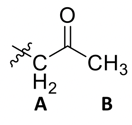
That leaves two methylene groups and one methyl group left. The low ppm range for these peaks are consistent with a chain of hydrocarbons.
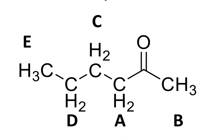
As you can see, each peak matches both in integration and splitting. The number of neighbors is also correct!
Here are the sources for Part 1 of this post:
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm
http://www.rsc.org/learn-chemistry/wiki/Introduction_to_NMR_spectroscopy
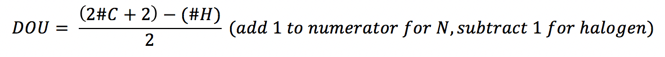

Comments