How to draw a molecular orbital diagram
Determine the atomic orbitals of your atoms
First, look at the periodic table: what are all the atomic orbitals of your valence electrons? (e.g. 2s, 3p)- Your valence electrons are the most involved in chemical bonding.
- Focus on your s and p orbitals—d and f are used less often.
- Include all valence orbitals—even the ones without electrons.
- You can find the shape of each atomic orbital in a textbook or online.
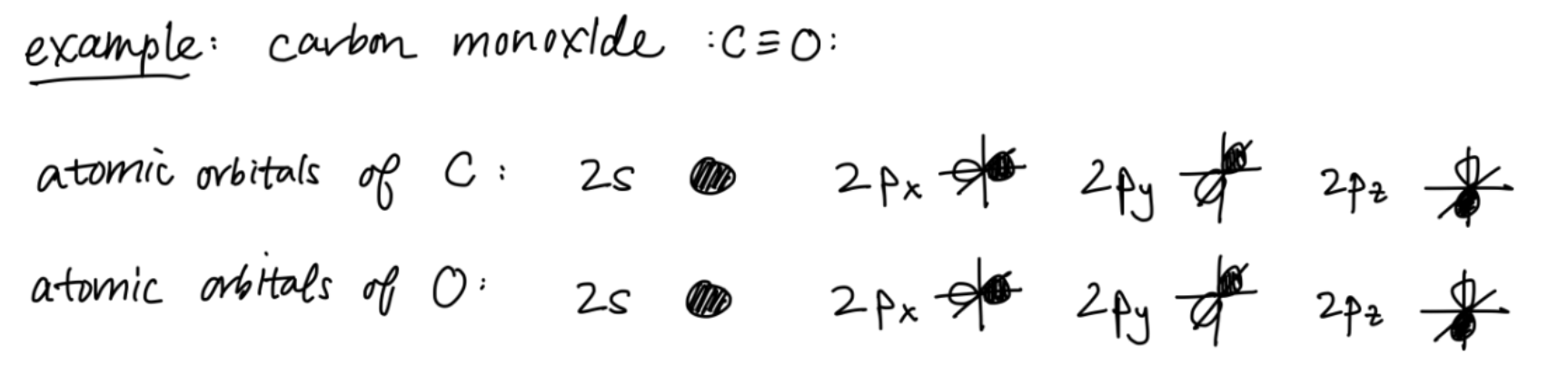
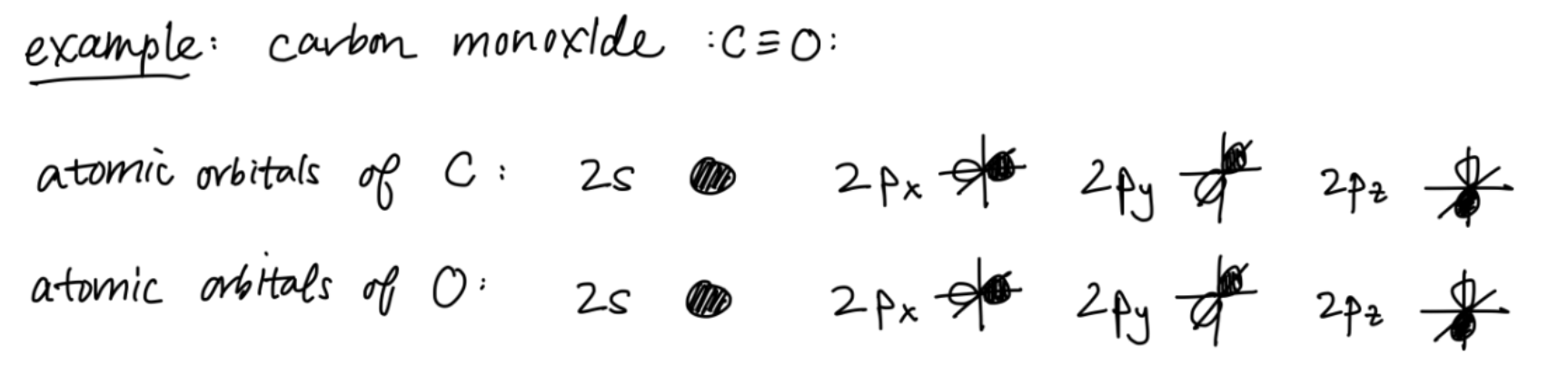 Next, draw one set of atomic orbitals for the first atom on the left, and another set for the second atom on the right.
Next, draw one set of atomic orbitals for the first atom on the left, and another set for the second atom on the right.
- Reference a table for the energies of the atomic orbitals (in a textbook or online) and take them into consideration when placing your orbitals.
- Fill in your atomic orbitals with the number of valence electrons of your respective atoms, following Hund’s rule and the Aufbau principle.
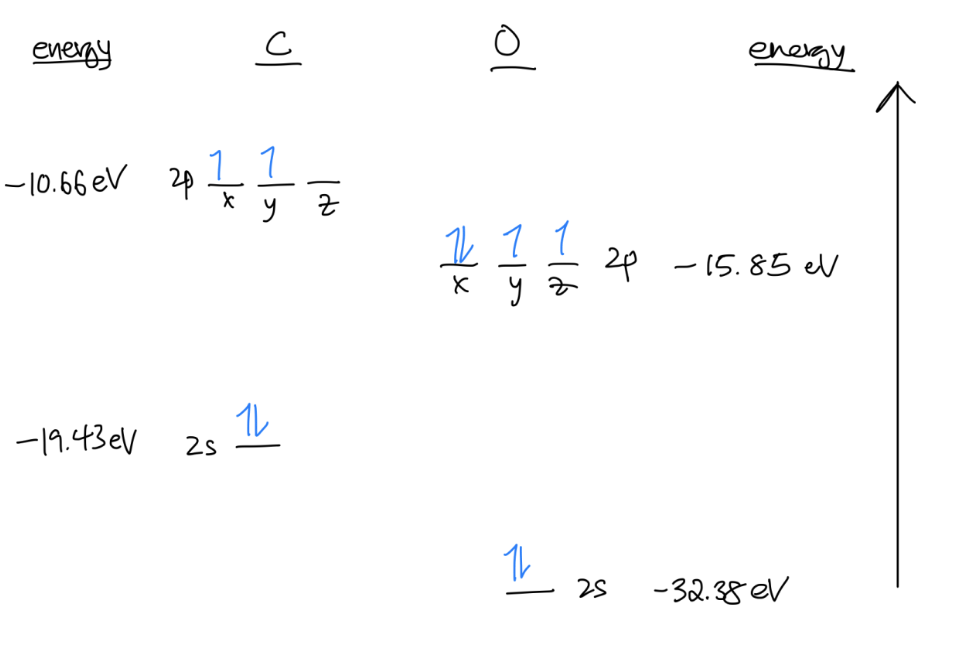
The # of atomic orbitals you have = the # of molecular orbitals you will make.
Determine the overlap of the orbitals
Now it is time to create your molecular orbitals. Remember, you will make the same # of molecular orbitals as the # of atomic orbitals. Consider:
- Symmetry (first priority) – Do the orbitals overlap?
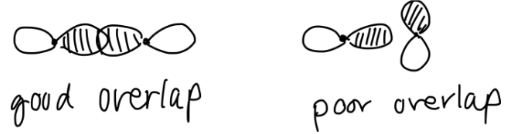
- Energy – Are the atomic orbital energies similar? Generally, atomic orbitals with an energy difference of less than 10-14 eV will interact to a significant degree (Miessler & Tarr).
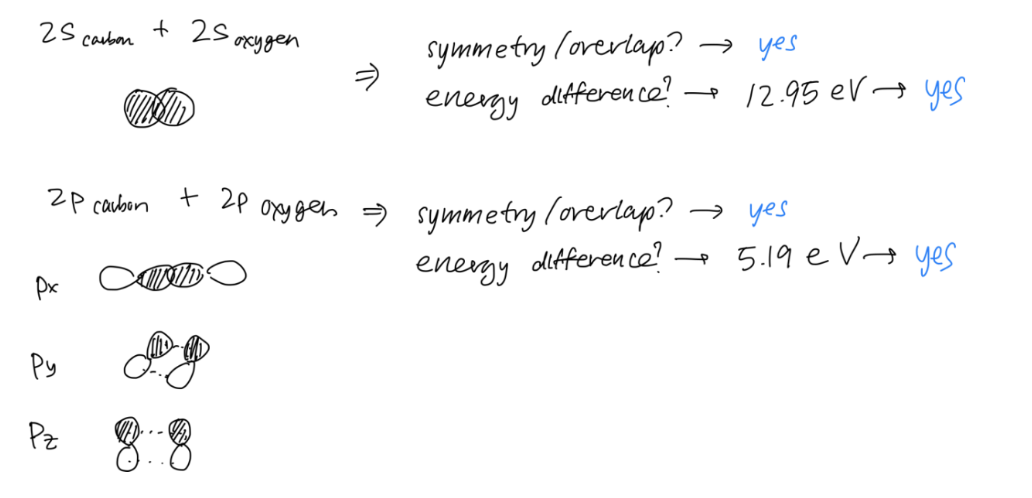
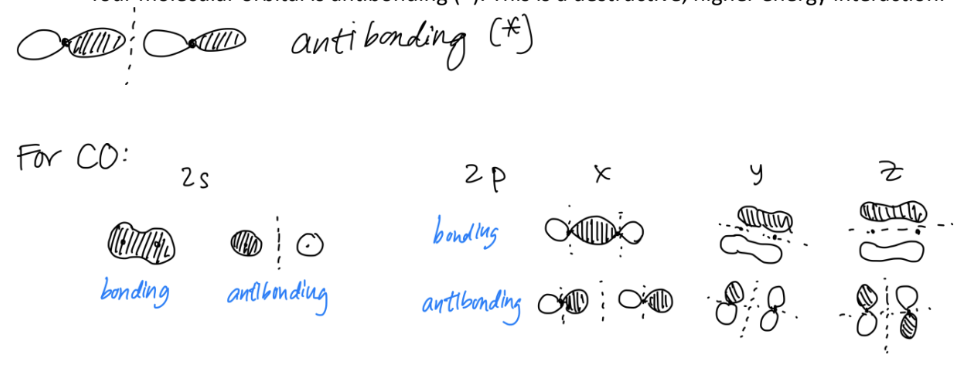
For each molecular orbital you create, you will create the ‘antibonding’ molecular orbital by inverting the sign of one of the atomic orbitals.

Now, list out all your molecular orbitals. Check that you have the same # of molecular orbitals as # of atomic orbitals.
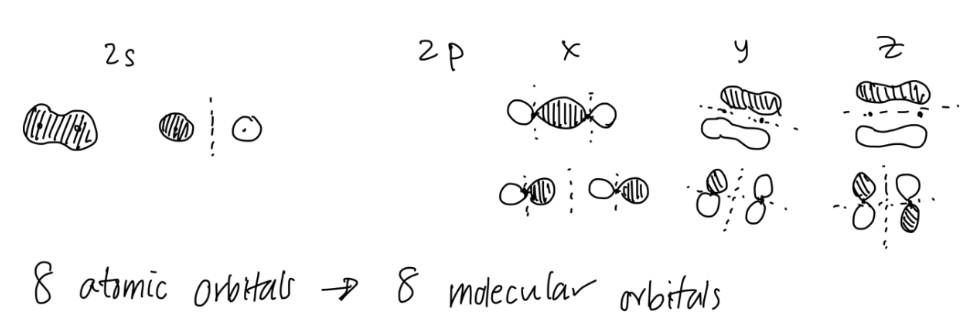
Assign bonding, antibonding, or nonbonding to the molecular orbitals
If your atomic orbitals overlap with the same sign, your molecular orbital is bonding (b). This is a constructive, lower energy bonding interaction.

If your atomic orbitals overlap with mixed signs, your molecular orbital is nonbonding (nb). This is a more neutral bonding interaction, and the electrons in this orbital typically appear as lone pairs.
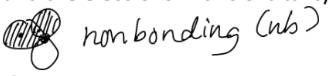
If your atomic orbitals do not overlap and have opposite signs, your molecular orbital is antibonding (*). This is a destructive, higher energy interaction.
Determine if your molecular orbital is a σ or π bond
Look down the bond axis.- If there is no sign change of the orbitals, it is a σ bond.
- If there is a sign change of the orbitals, it is a π bond.
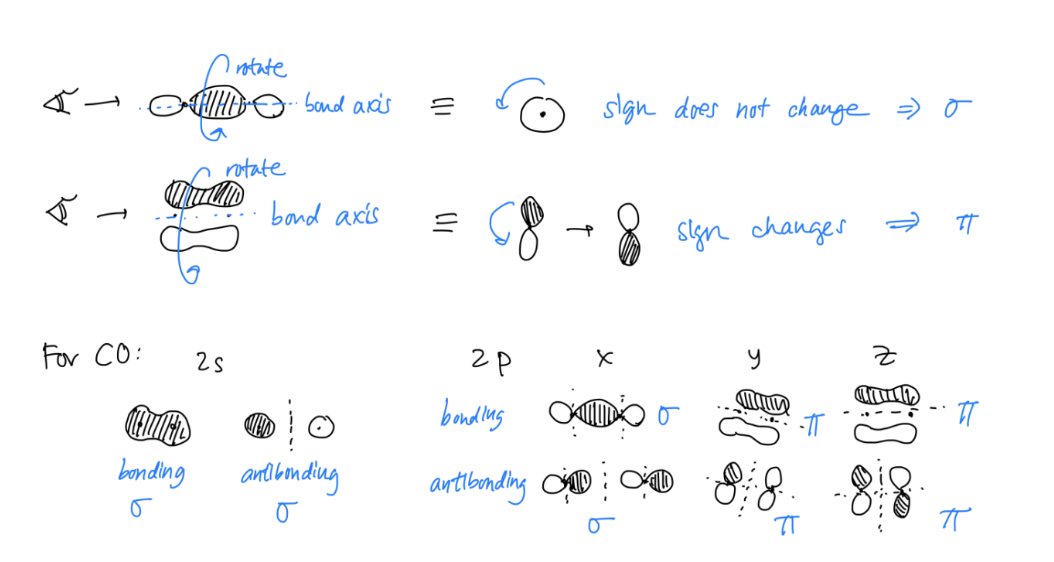
Order your molecular orbitals in the diagram
Consider:
- Energy levels of the atomic orbitals that formed the molecular orbital. Lower energy orbitals will be lower on the diagram.
- Bonding, nonbonding, or antibonding. Bonding interactions are more stable and lower in energy, so they will be lower on the diagram. Antibonding interactions are high energy interactions and will be higher on the diagram.
- σ or π. σ bonds have fewer nodes, and will generally be more stable, lower in energy, and lower on the diagram than π bonds. Conversely, σ antibonds will be more unstable, higher in energy, and higher on the diagram than π antibonds.
General energy order: σb < πb < π* < σ*
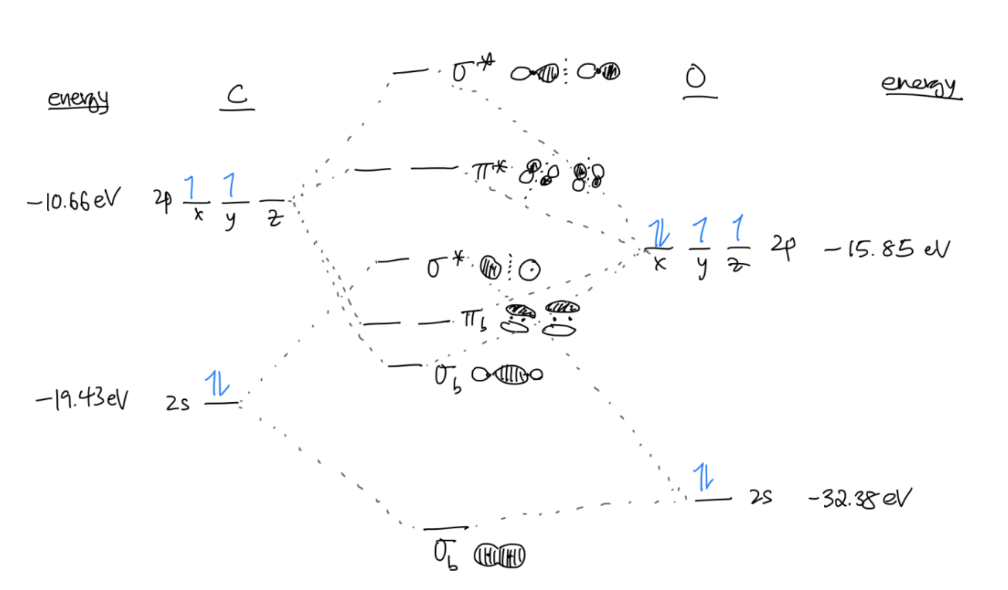 Fill in the electrons in your diagram with the total number of valence electrons from both atoms, following Hund’s Rule and the Aufbau principle.
Fill in the electrons in your diagram with the total number of valence electrons from both atoms, following Hund’s Rule and the Aufbau principle.
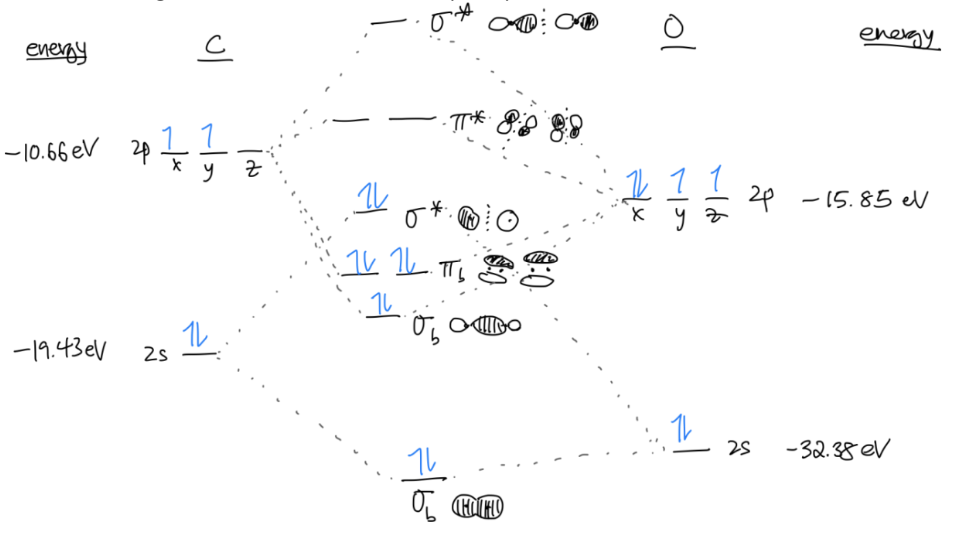
Congrats! You have your molecular diagram.
So, what can a molecular orbital diagram tell you?
1. Bond Order
Bond order gives you an idea of the strength of the bond between two atoms. As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc.
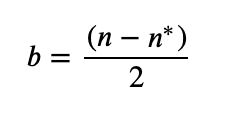
b = bond order
n = # electrons in bonding orbitals
n* = # electrons in antibonding orbitals
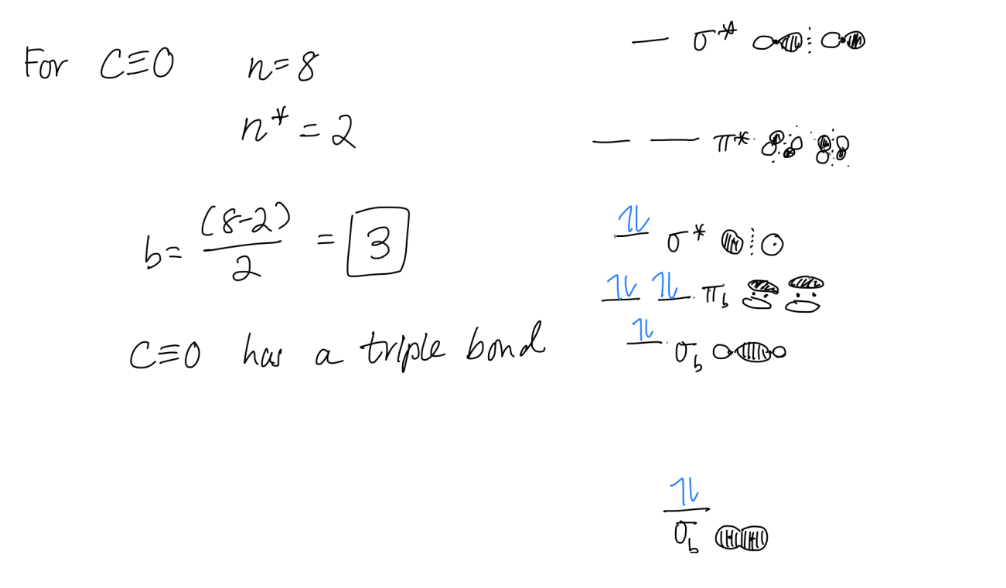
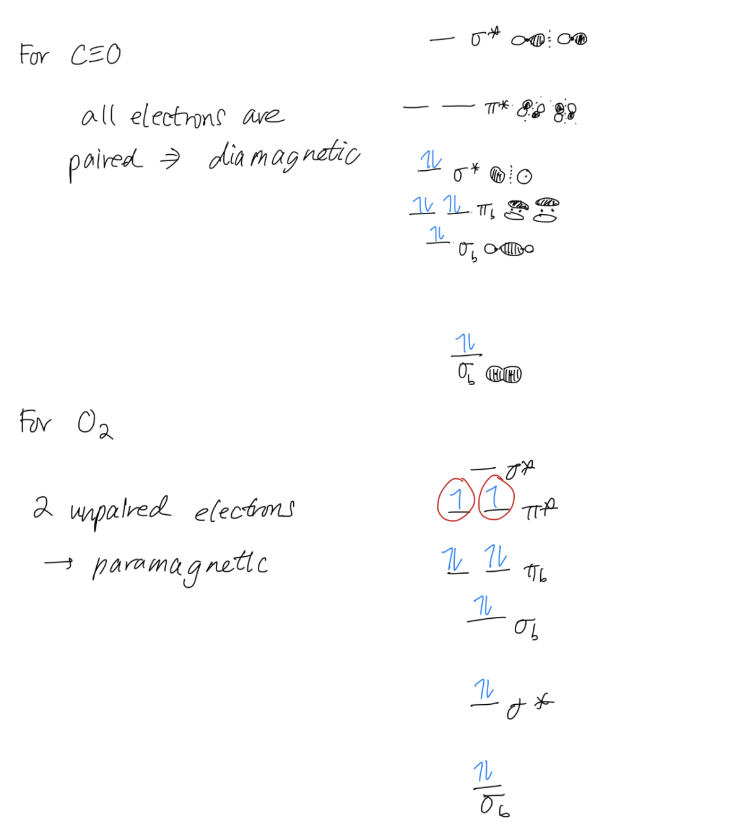
2. Magnetism
Magnetism will tell you whether your compounds will be attracted to or repelled by a magnetic field.
Take a look at your MO diagram.
- If all your electrons are paired: your compound is diamagnetic. Diamagnetic compounds are repelled by magnetic fields.
- If you have unpaired electrons: your compound is paramagnetic. Paramagnetic compounds will be attracted to magnetic fields.
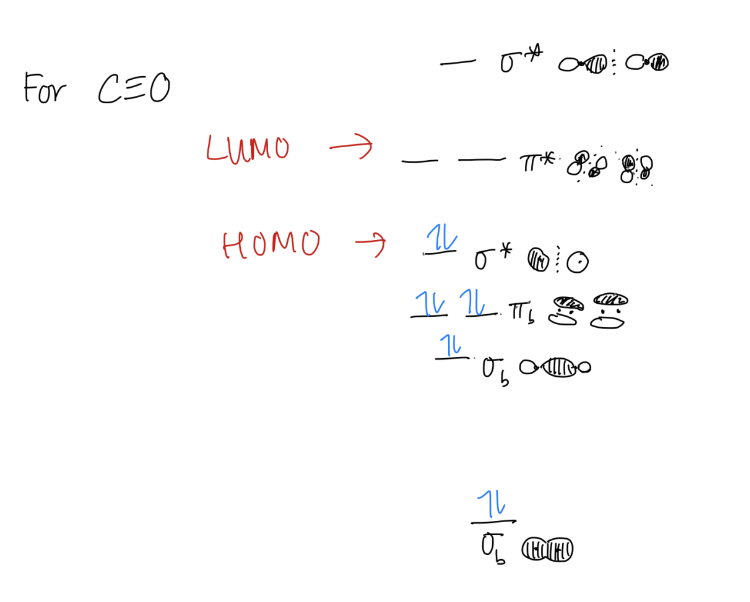
3. HOMO/LUMO
Your molecular orbital can tell you what your highest occupied molecular orbital (HOMO) and your lowest unoccupied molecular orbital (LUMO) is. This can be useful for predicting reactions between two molecules.
 Please Note:
Please Note:
This is a simple, mostly qualitative analysis of the molecular orbital diagram. To determine the ordering of more ambiguous molecular orbitals, or for more rigorous modeling, spectroscopy techniques or computational chemistry software such as Gaussian can be used.


Comments