Intermolecular forces (IMFs) are “electrostatic” interactions between molecules – a result of all the charges floating around and interacting in the system. IMFs influence the properties of substances that we can observe and interact with – for example, the phase of the substance or its boiling point.
IMFs are generally divided into two categories: van der Waals forces (permanent dipole-dipole interactions and London dispersion forces between temporary (instantaneous) dipoles) and hydrogen bonding.
1 – What is a permanent dipole? How do they interact?
Atoms interact and form bonds because they want to become “happier” – that is, more stable. This really boils down to electronegativity – how much an atom wants to get more electrons. If 2 atoms have similar electronegativities, the most stable situation for both atoms is to roughly share the electron density between them. If 2 atoms have very different electronegativities, the most stable situation would be for the less electronegative atom to give its electron density to the more electronegative atom.
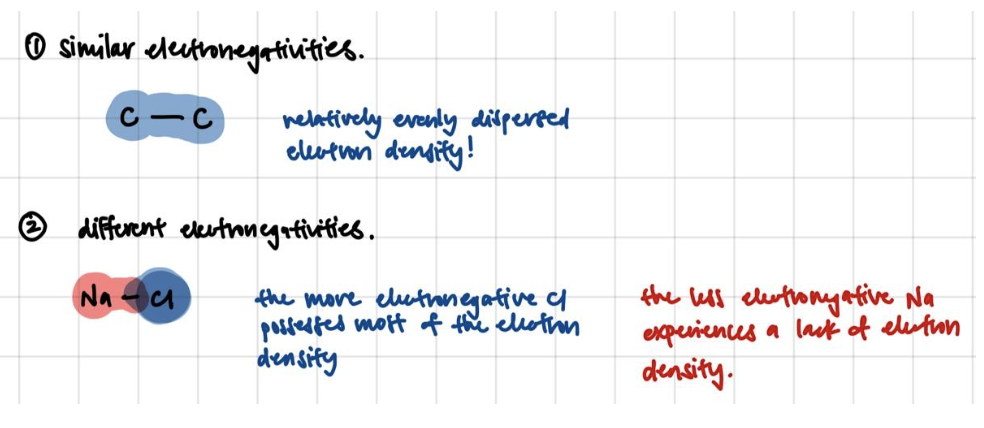
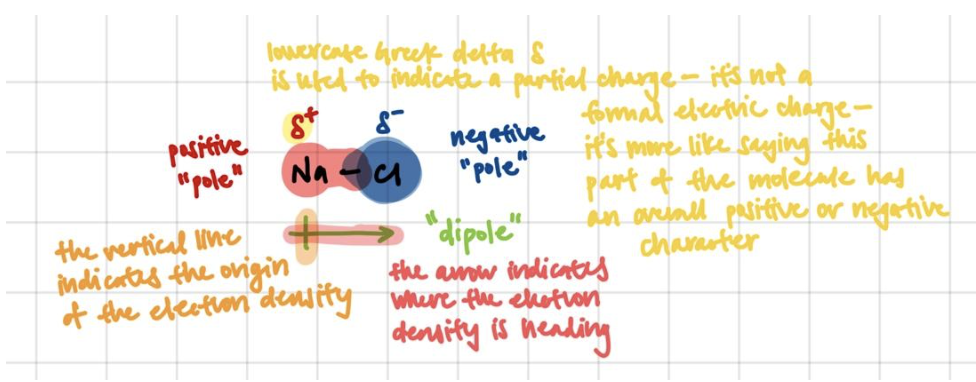
That pull of electron density from a less electronegative atom to a more electronegative atom results in a “dipole”.
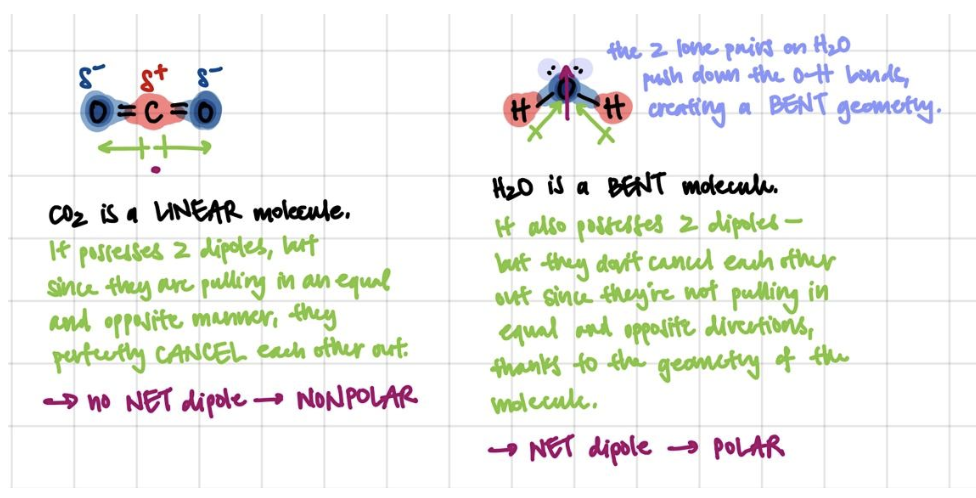
The presence of dipoles in a molecule doesn’t mean that the molecule is polar! Dipoles can actually cancel each other out, and molecules must have an overall, net dipole moment in order to be considered “polar”. Whether or not the dipoles cancel is dependent on the geometry of the molecule, which can be determined based on VSEPR rules.
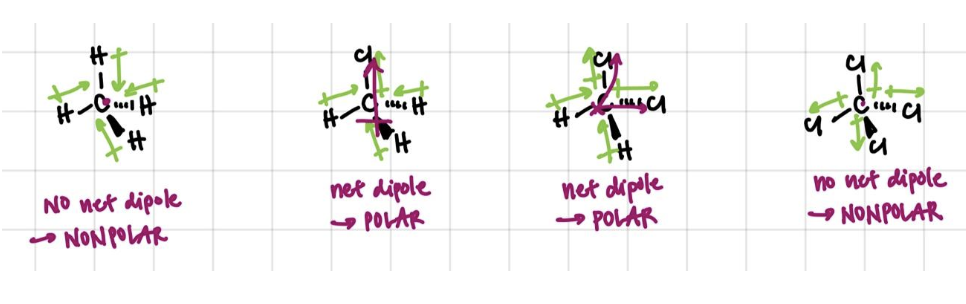
We can think more about how dipoles can cancel each other out by starting with methane and sequentially substituting H with Cl:
Permanent dipoles will attract each other like magnets – the δ+ part of one molecule to the δ- part of another. These are the van der Waals dipole-dipole interactions.
2 – What is a temporary (instantaneous) dipole? How do they interact?
Electrons are not static – they’re constantly moving around within the electron cloud densities around the molecule. That’s why we often refer to “electron density” rather than directly saying “electrons”.
As electrons are moving around, it’s natural for there to be instants in which there is a temporary concentration of electrons. Imagine shaking around a box filled with marbles – if you took a million snapshot images of the box, you’d observe some photos in which all the marbles are concentrated at one part of the box. These temporary densities and lack thereof of electrons (marbles) create temporary (instantaneous) dipoles. Because these temporary dipoles occur simply as a result of electrons moving around and not due to any other more specific structure or character of the molecule, any molecule can experience temporary dipoles.
These instantaneous dipoles in turn can create induced dipoles in other molecules (polar or nonpolar). It can be helpful to think about electron densities as fluffy clouds with negative charge that can be repelled by other negative charges. If the δ- part of a Molecule A possessing an instantaneous dipole approaches the fluffy density cloud of Molecule B, the cloud will be repelled and Molecule B will now experience an induced temporary dipole. Molecule A and Molecule B will then experience a temporary dipole-temporary dipole interaction, termed a “London dispersion force”. Since all molecules can experience temporary dipoles, all molecules experience London dispersion forces. Temporary dipole interactions are the weakest of the IMFs.
Different molecules will experience different degrees of inducibility, depending on the “fluffiness” of the cloud. The more tightly a nucleus holds on to its electrons, the less fluffy the cloud and the less inducible the dipole. How tightly a nucleus holds on to its electrons is related to how far the electrons are from the nucleus – farther electrons experience less pull from the nucleus thanks to electron shielding and the inverse square nature of Coulomb’s law.
3 – What is hydrogen bonding?
Hydrogen “bonding” is a bit of a misnomer. It is referred to as a “bond” because it is such a strong interaction, but it is still an interaction, not a bond.
Only nitrogen, oxygen, and fluorine are electronegative enough to participate in these types of reactions. However, the presence of N, O, and F are not sufficient. For two molecules to participate in hydrogen bonding,
- the first molecule must have a hydrogen bound to N, O, or F; and
- the second molecule must have a lone pair of electrons on N, O, or F.
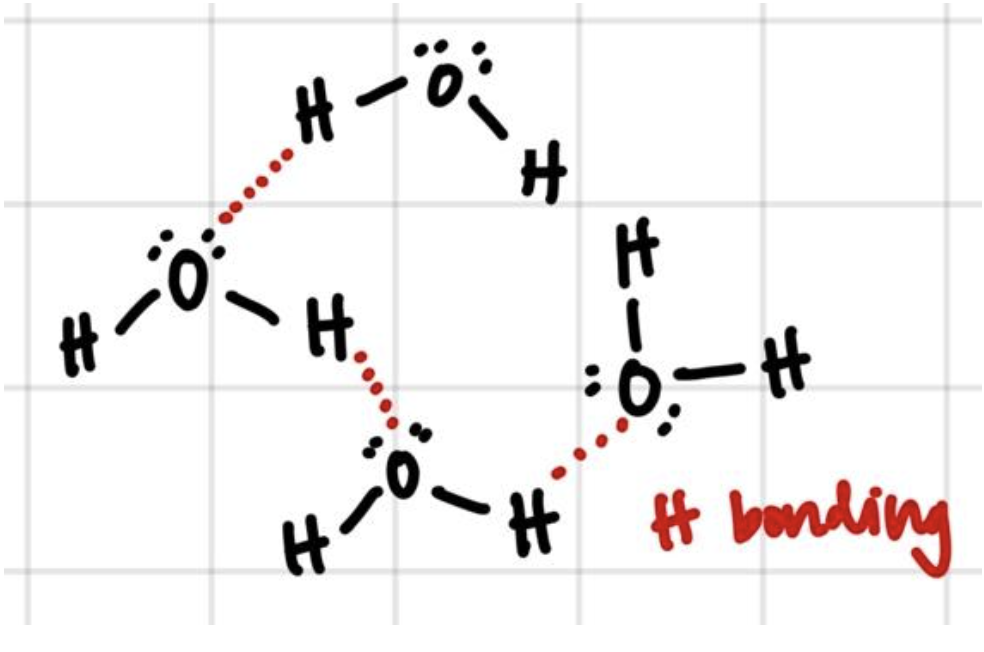
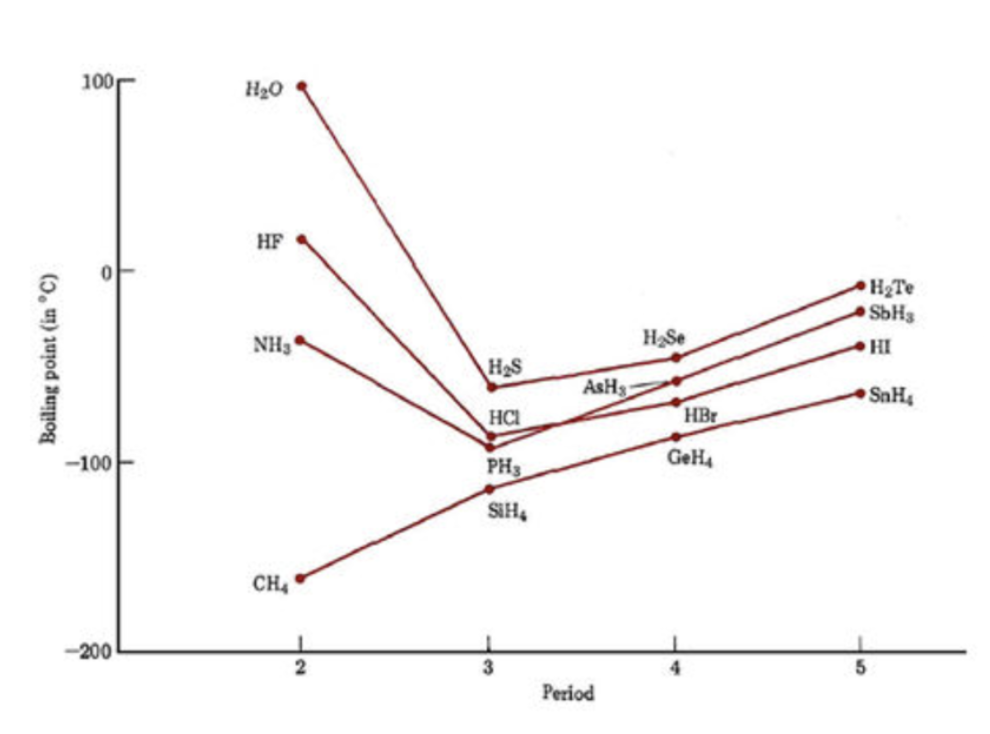
Because H bonding is such a strong interaction, it can result in large changes in properties such as boiling point. This is, for example, why water has a much higher boiling point than we would expect for a dihydrogen chalcogen. H2O is the only dihydrogen chalcogen that can participate in hydrogen bonding, and that interaction is strong enough that it significantly increases the amount of energy it requires to break the IMFs and boil the water.

Comments