Welcome to Part 2 of our foray into lab techniques! In my last post, we discussed the basic principles behind gel electrophoresis. In this post, we’ll build off the principles behind gel electrophoresis and talk about its cousin, SDS-PAGE, with a focus on how it is different from gel electrophoresis. If you need a refresher on gel electrophoresis, check out Part 1!
SDS-PAGE: Just a Gel for Protein
SDS-PAGE is an acronym for a long name that you don’t need to remember. The “GE” in SDS-PAGE stands for “gel electrophoresis,” meaning it works just like the regular gel we discussed in Part 1. The main difference here is that gel electrophoresis separates DNA, while SDS-PAGE separates protein. You can use the “P” in SDS-PAGE to help you remember that it separates protein.
Like gel electrophoresis, the purpose of SDS-PAGE is to separate molecules (in this case protein) by size.
A Reminder of Our Basic Rules:
Recall our two basic rules of the universe that everything, including SDS-PAGE, must follow:
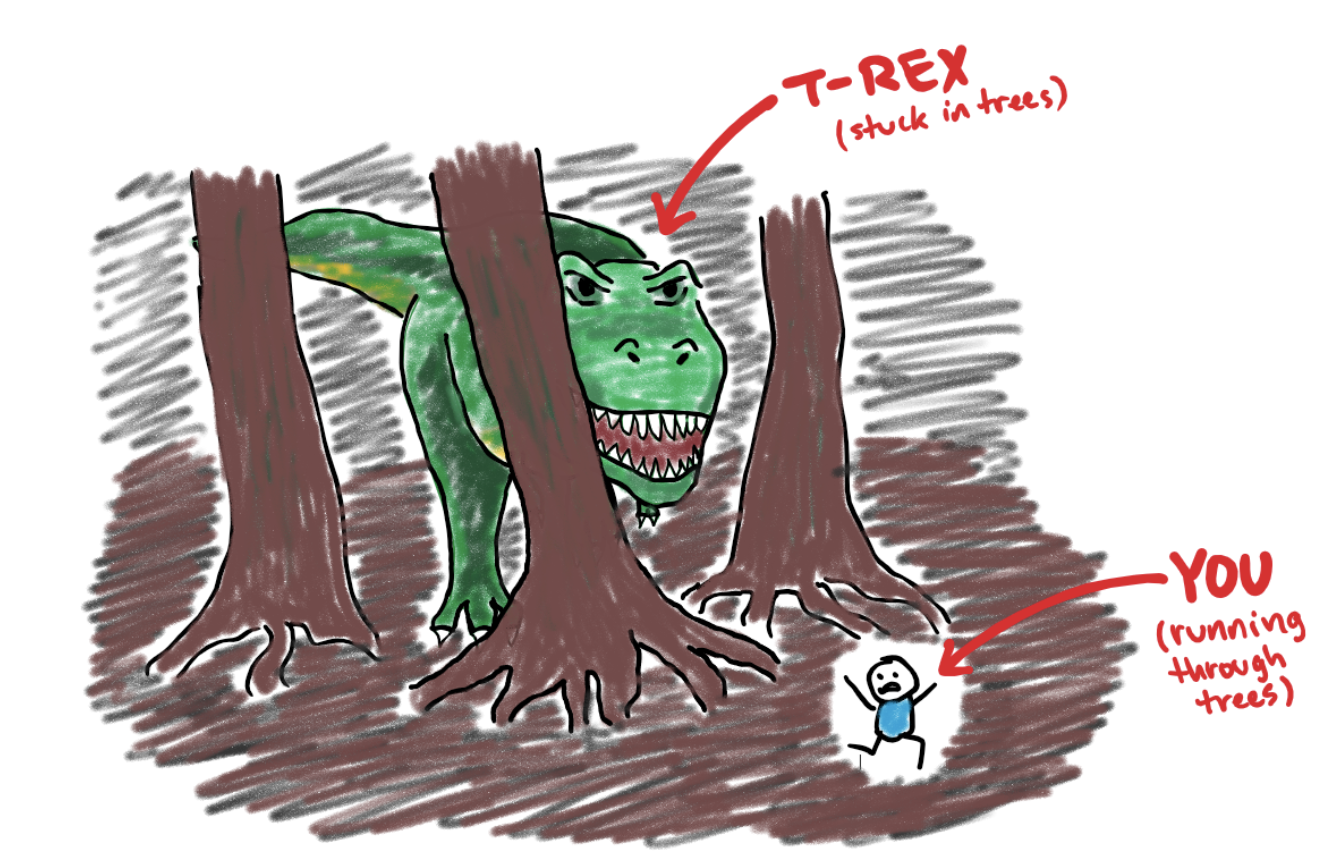
Rule #1: Big dinosaurs can’t fit through small holes
Remember that if you’re ever chased by a T-rex, your best strategy is to run into a dense forest with giant trees. The T-rex, being very big, will have a hard time fitting through the trees, and will thus be slowed down. You, meanwhile, can easily slip between trees and get away. Similarly, any gel, including the one used in SDS-PAGE, is a dense matrix that will slow down larger molecules while letting smaller molecules move relatively unimpeded.
Rule #2: Opposites attract
Specifically, positive and negative charges attract. Easy, right?
But if SDS-PAGE has the same purpose, why can’t you use a regular gel?
Unlike DNA, which intrinsically has a negative charge, proteins can be of any charge. Proteins are also folded into different shapes: some are bulky, some are streamlined. These two aspects affect travel speed through a charged gel and can mess up your result. That’s where the SDS comes in.
SDS is a negatively charged material that coats proteins. In SDS-PAGE, the proteins are denatured (i.e. unfolded) and then coated with SDS which gives each protein a uniformly negative charge along its entire length – this is called having a uniform charge-to-mass ratio. Once your proteins have been coated with SDS, they start acting basically like DNA molecules in a gel, and will move through the gel based on size just like we described above. Protein size is measured in kilodaltons (kDa), so here’s what that might look like:
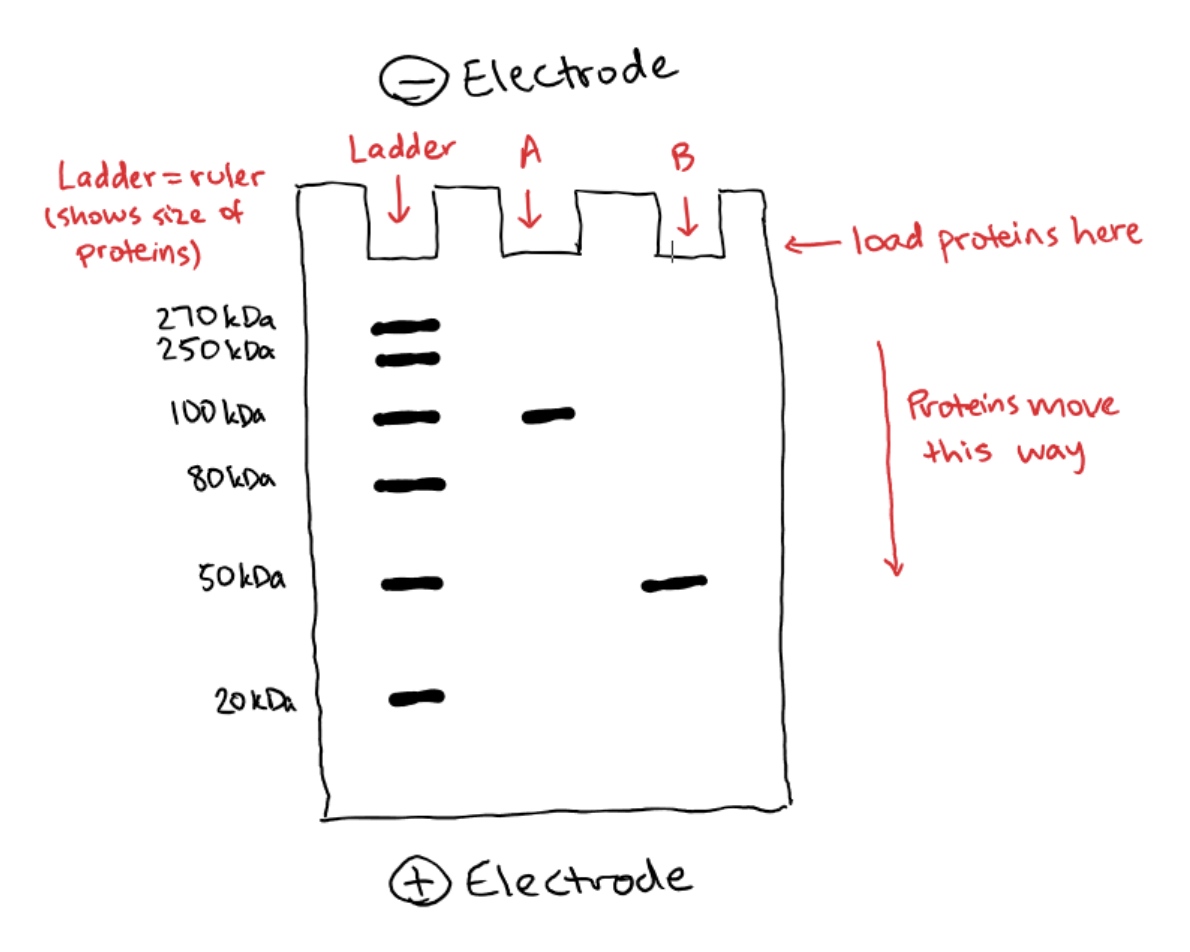
Once again, we have a ladder which is composed of several proteins of known sizes. This helps us estimate the size of our samples. In this case, Protein A is larger at about 100 kiloDaltons (kDa) because it not moved very far from the well it started in. Protein B, on the other hand, moved quite a distance and we can see that it is smaller at about 50 kDa.
In summary, SDS-PAGE separates proteins by size. It involves unfolding proteins and coating them with negatively charged SDS so that they behave just like DNA in a regular gel.
Take Home Points
What is the purpose of SDS-PAGE?
SDS-PAGE is used to separate proteins based on size.
How does it work?
SDS-PAGE works by taking proteins, coating them in negatively charged SDS, and pushing them through a dense gel toward a positive pole. Remember our basic rules? Opposites attract.
How do you interpret the results?
Bigger proteins move slower, so they end up closer to where they started. Smaller proteins move further along the gel. Remember, the gel is the forest and big dinosaurs can’t fit through small holes.
The road to medical school is long, and the MCAT is one of its most formidable challenges. You will be relieved to know that what you learned in your premedical courses is actually on the test. But studying for the MCAT is more about taking that knowledge stored way back there in the nooks and crannies of your mind, bringing it to the fore, and then learning to twist and stretch it in the ways the MCAT tests. In reality, studying for the MCAT is no more (or less) difficult than spending late hours on a physics problem set or an entire weekend on an organic chemistry lab report. Just like these other tasks, the MCAT requires endurance and follow-through, but it becomes significantly more manageable when you work with a Cambridge Coaching MCAT tutor to apply a structured, systematic, and strategic approach to your studying.
Anyone can study hard - but the real key to MCAT success is learning to study smart. So, while all forms of MCAT preparation require you to crunch a lot of material, we focus on helping you to make strategic choices about your areas of focus at every step of the game. Each Cambridge Coaching tutor is a highly-skilled manager of your personal study process. He or she will do more than just target your weaknesses - your tutor’s goal is to identify the sections where you have the greatest potential for improvement, and teach you to wring every last point from them by creating the roadmap for your studying, and helping you stick to it. Right from the start, your tutor will create a customized syllabus for you, and will then modify that syllabus as needed.

Comments