-4.png?width=1080&name=Title_%20How%20to%20Study%20Efficiently%20for%20Hours%20On%20End%20(With%20the%20Help%20of%20a%20Tomato)-4.png) Although there are lots of tricks that people use to write chemical formulas for ionic compounds, the best way to do it is to really understand what’s going on – that way, you’ll never use a shortcut that doesn’t work.
Although there are lots of tricks that people use to write chemical formulas for ionic compounds, the best way to do it is to really understand what’s going on – that way, you’ll never use a shortcut that doesn’t work.
Remember that an ionic compound is made up of a positively charged ion and a negatively charged ion. Use the periodic table below to remind yourself which groups of elements tend to form which charges:
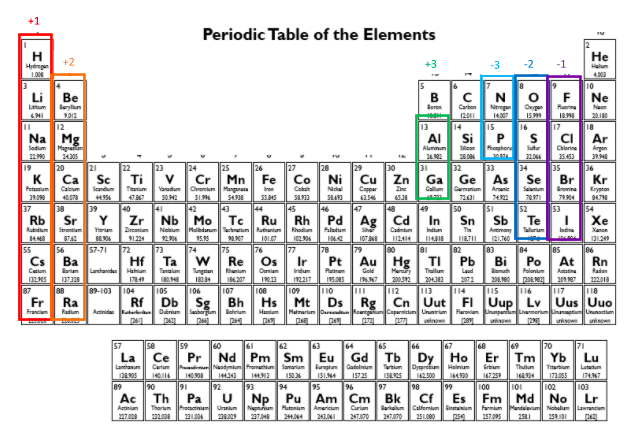 Let’s say we wanted to write the formula for lithium nitride. From the periodic table, we see that lithium forms +1 ions, and nitrogen forms -3 ions. In any ionic compound, we need the total number of positive charges to be equal to the total number of negative charges so that the compound is neutral (uncharged) overall. So, how many lithium (+1) and how many nitrogen (-3) ions do we need?
Let’s say we wanted to write the formula for lithium nitride. From the periodic table, we see that lithium forms +1 ions, and nitrogen forms -3 ions. In any ionic compound, we need the total number of positive charges to be equal to the total number of negative charges so that the compound is neutral (uncharged) overall. So, how many lithium (+1) and how many nitrogen (-3) ions do we need?
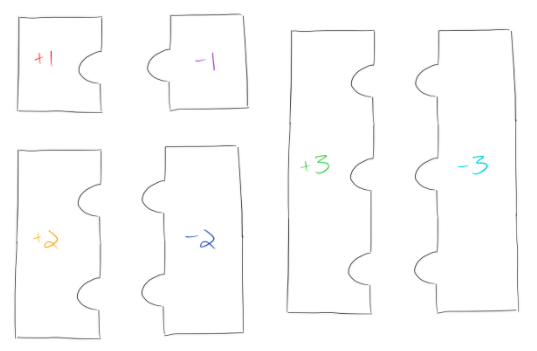
We’ll use puzzle pieces to represent our ions. One indentation in a puzzle piece represents one positive charge, and one part sticking out of a puzzle piece represents one negative charge:
 So, if we want to make a nice, complete puzzle out of lithium (+1) and nitrogen (-3) puzzle pieces, we’ll need 3 lithium pieces (each having a charge of +1) and 1 nitrogen piece (which has a charge of -3):
So, if we want to make a nice, complete puzzle out of lithium (+1) and nitrogen (-3) puzzle pieces, we’ll need 3 lithium pieces (each having a charge of +1) and 1 nitrogen piece (which has a charge of -3):
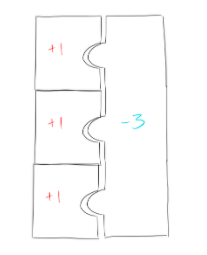
Because we have 3 lithium pieces and 1 nitrogen piece, our formula is Li3N.
Let’s do another example: what is the formula for calcium oxide? Looking at the periodic table above, you see that calcium forms a +2 ion, and oxygen forms a -2 ion. Our completed puzzle, then, will look like this:
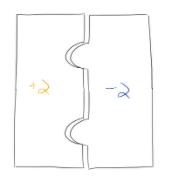
Since we have one calcium piece and one oxygen piece, our formula is CaO.
Here’s a third, more challenging example: what’s the formula for aluminum sulfide? From our periodic table, you can see that aluminum will have a +3 charge, and sulfur will have a -2 charge. These charges are a little harder to balance, but if you play around with the puzzle pieces, you’ll get a completed puzzle that looks like this:
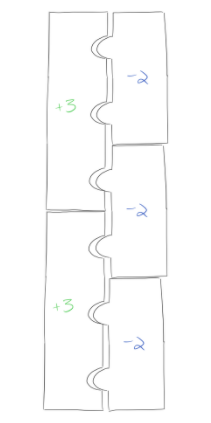
Since we need 2 aluminum pieces (each with a +3 charge) and 3 sulfur pieces (each with a -2 charge) to create a compound that’s uncharged overall, the formula for this compound is Al2S3.
Using puzzle pieces, you can figure out the formula for any ionic compound, no matter what charges the ions have. Just keep adding puzzle pieces until you get a complete puzzle. This strategy to balance the charges will never fail!
Chemistry is one of our most popular subjects and we have helped countless students conquer chemistry. Our chemistry tutors are doctoral candidates and PhDs. Our team also includes MD candidates, MDs, and MD-PhDs. Most of our tutors have served as teaching assistants for hundreds of undergraduates, many of whom are encountering chemistry for the first time. Our tutors can work with students of all ages to deepen their study of chemistry at any level.
Many of our tutors specialize in teaching introductory courses, and making chemistry accessible to their students. Others specialize in preparing students for standardized tests such as the Chemistry SAT II, AP, or GRE, as well as the MCAT. We have access to the standard text books, and many of our students choose to work with a tutor in advance of, or concurrent with, challenging high school or college chemistry classes. We can easily combine chemistry tutoring with tutoring in biology, physics, and mathematics. We do our best to match students with tutors who are most appropriate to their level and learning style.
Need help with your chemistry studies? Check out some of our other helpful blog posts below!
How to begin preparing for the SAT subject test in chemistry
Formal Charge: What They Didn’t Tell You in your General Chemistry Class
Organic Chemistry: This Subject Gives You Alkynes of Trouble!

Comments