While Organic Chemistry makes up a smaller portion of the MCAT, understanding the distinctions between types of organic reactions is essential. This will outline several foundational strategies for tackling chemistry on the MCAT, without pure memorization. Having a strong comprehension of organic reactions will allow you to save valuable time on the test and narrow down your multiple-choice options.
Key Player Identification
Our first step when tackling an unfamiliar reaction with multiple reagents is to determine which component is the nucleophile versus electrophile.
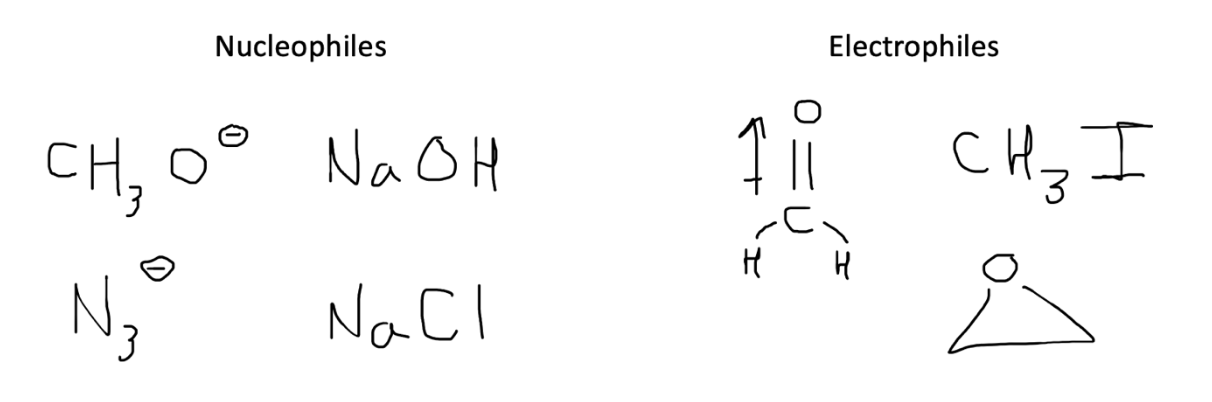 A nucleophile will typically be an atom or molecule with a surplus of negative charge, namely an extra electron pair. This nucleophile is looking to attack a more electrophilic, or electron deficient, atom. Common electrophiles include those bonded to electronegative or electron-withdrawing groups or atoms participating in a dipole.
A nucleophile will typically be an atom or molecule with a surplus of negative charge, namely an extra electron pair. This nucleophile is looking to attack a more electrophilic, or electron deficient, atom. Common electrophiles include those bonded to electronegative or electron-withdrawing groups or atoms participating in a dipole.
Movement of electrons
For the MCAT, it is essential to identify these two key players first, so that we can track the flow of electrons and eventually solve with our most stable configuration. I always found it helpful to draw an arrow from the nucleophile to the electrophile, showing the movement of electrons as the nucleophile “attacks” the electrophile with electrons. To remember this, I think that the nucleophile would love a nucleus or positive charge, so it gives its electrons to another atom. By giving away electrons, the nucleophile is less negative, therefore gaining positivity.
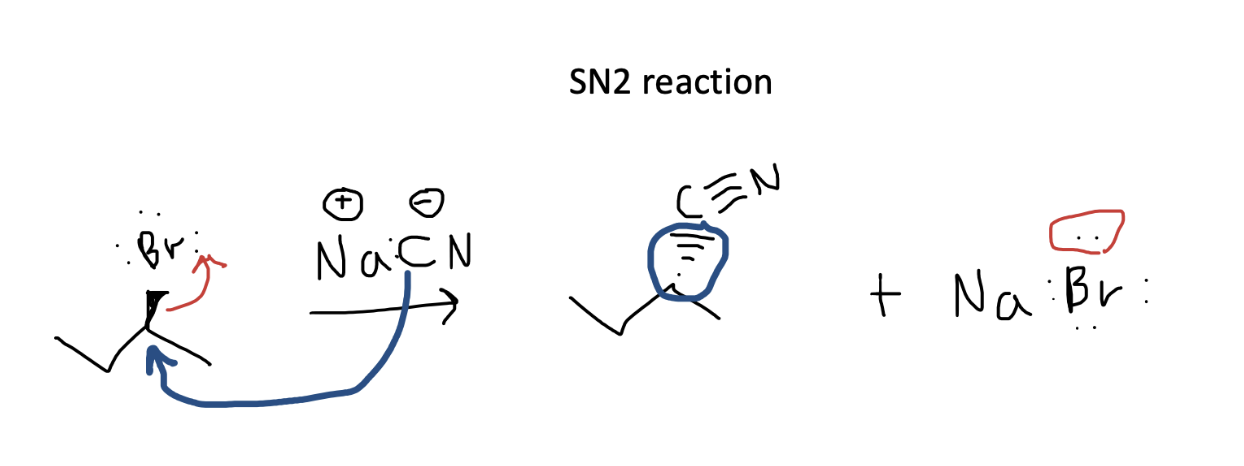
In this simple SN2 reaction, we can see that the nucleophile, the carbon in the nitrile which has a negative charge, attacks the carbon attached to the electron-withdrawing bromine. It is helpful to track both electron movements separately, so you can ensure your answer contains the appropriate number of electrons (none are lost!). In blue, we have the carbon’s electrons attacking the bromoalkane and forming a new carbon-carbon bond. The electrons from the original carbon-bromine bond, move onto bromine giving it a full set of 8 electrons and a new negative charge. Don’t forget that in SN2 reactions, there is a backside attack so the stereochemistry reverses in the new bond formed by the attack.
Once the major movement of electrons can be predicted, we want to establish what the most stable form will be. Electrons move to the most stable configuration and seek to balance charge differences, with a few exceptions. By keeping this in mind, you can eliminate answer choices that would not realistically occur and, of course, any that break the octet rule.
There are, of course, a few reactions that you should know well as they occur frequently in biochemistry. These include the dehydration reactions and others involved in the citric acid cycle. On the exam, however, if you are pressed for time, you can use your deep understanding of electrophiles and nucleophiles to eliminate answer choices and find the most reasonable solution quickly!
Comments