If we had to pick one go-to answer to explain as many things as possible in biochemistry, it would probably be “the hydrophobic effect.” It’s responsible for protein structure and function, cell membrane organization, and the distribution of drugs and metabolites. It’s often an important consideration in drug design. But what is it, and how does it work?
Chemistry review: polar and non-polar molecules
Some covalent bonds are polar, meaning that, although the two atoms share electrons, one atom is more electronegative and exerts a greater “pull” on the electrons. Even though there are no formal (full) charges, this uneven pull creates a partial negative charge on one atom and partial positive on the other (as the electron density is unevenly distributed). For example, in the figure below, oxygen atoms have partial negative charges, and the carbon and hydrogen atoms have partial positive charges. This partial charge distribution, or bond dipole, constitutes a polar bond.
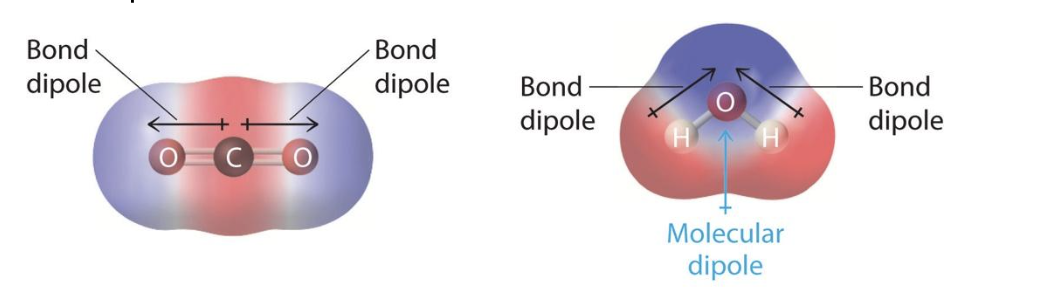
Figure 1: Representation of the electron density in CO2 and H2O, with regions of partial negative charge (more electron density) in blue and partial positive regions in red.
When a molecule contains one or more bond dipoles that do not cancel each other out, it’s a polar molecule. Molecules that have no polar bonds or have polar bonds that cancel each other out (like carbon dioxide, above), are non-polar. Because water’s hydrogen-oxygen bonds are oriented 104.5° apart, they do not cancel each other out. Water is therefore a polar molecule.
Water is a polar solvent that “likes” polar molecules
Polar molecules are attracted to one another through their partial positive and partial negative regions, which can significantly impact their behavior. Water molecules experience a special kind of interaction called a hydrogen bond: an attraction between 1) a partially negative atom with a lone pair of electrons and 2) a partially positive hydrogen atom in a different polar bond. Hydrogen bonds are particularly strong for non-covalent interactions. Water molecules form a shifting network of hydrogen bonds that drive water’s behavior as a solvent.
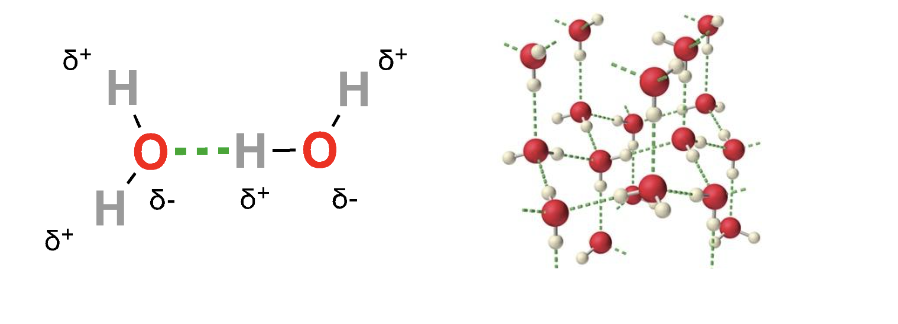
Figure 2: Left: A hydrogen bond (green) between two water molecules. Right: hydrogen bonding network.
Polar molecules, which are typically capable of hydrogen bonding, interact easily with water. They are coated, or “solvated,” by water molecules engaging in hydrogen bonding and dissolves easily. We therefore call polar molecules hydrophilic, or “water-loving.” Non-polar molecules are hydrophobic: they cannot hydrogen bond with water molecules, dissolve poorly (or not at all) and at a molecular level aren’t well solvated. Because of their large size, some biomolecules (like phospholipids) have distinct hydrophobic and hydrophilic regions. We call these molecules amphiphilic.
The hydrophobic effect
Because hydrophobic molecules can’t form hydrogen bonds, water molecules don’t “like” associating with them as much as they “like” associating with each other. They exclude hydrophobic molecules, pushing them together in order to minimize how much the water molecules have to interact with them.1 The hydrophobic effect is the tendency of different hydrophobic molecules, or the hydrophobic regions of a large macromolecule, to cluster together in a way that minimizes their interaction with water.
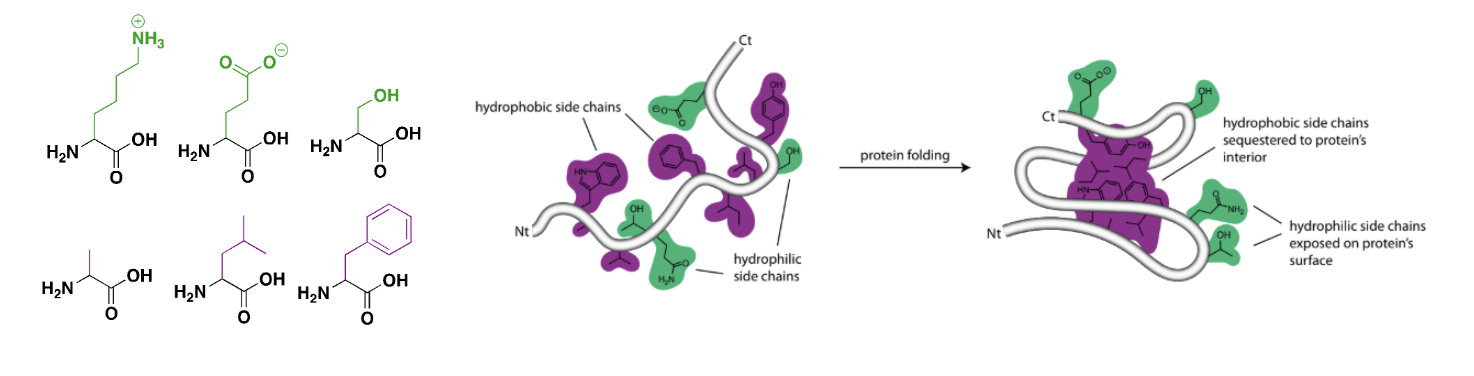
Figure 3. Left: Some amino acids have hydrophilic side chains (green) and others have hydrophobic side chains (purple). Right: In water, polypeptides assemble in a way that buries these hydrophobic side chains in the core of the folded protein. Hydrophilic residues remain exposed to water at the protein’s surface.
Where it comes up
The hydrophobic effect dictates structure--and therefore function--for many proteins. It also impacts protein-protein interactions and the binding of co-factors, regulators, substrates, and inhibitors, which often bind within hydrophobic pockets. Hydrophobicity and hydrogen bonding are important considerations in developing drugs and therapeutic molecules, which need to interact with specific proteins in specific ways.
The hydrophobic effect comes up in many other contexts throughout biochemistry: the organization of cell membranes; the distribution of metabolites, drugs and other molecules; and the structure and behavior of DNA and RNA. It plays a role in disease as well: for example, causing protein aggregation (misfolded clustering) in neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Understanding the hydrophobic effect will help you understand a wide variety of biochemical processes that are fundamental to cellular function, metabolism, disease onset, drug design, and more!
1 In more technical terms, there is an unfavorable free energy associated with these interactions. Hydrophobic molecules disrupt hydrogen bonding between water molecules and impose a higher degree of order, as the surrounding water molecules form a cage-like structure around the hydrophobic molecule. This decreases the water molecules’ mobility and therefore their entropy, which is unfavorable. (In some cases, the contributions shift to be more enthalpic than entropic, but this is the most common understanding.) Sequestering the hydrophobic molecules/regions of a large molecule together in a way that minimizes their contact with water molecules also minimizes this free energy penalty; as a result, this process is favorable and occurs spontaneously.


Comments