In this blog post, we will complete the following example problem:
Draw the chair conformation of α-d-galactopyranose
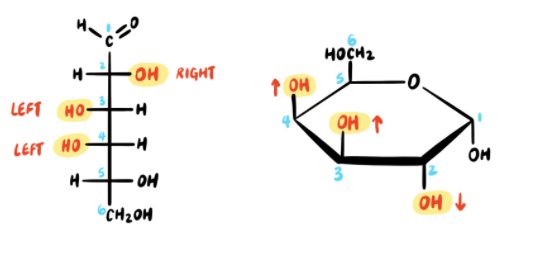
First, we must draw the Haworth structure of α-d-galactopyranose from the Fischer projection of d-galactose.
For the MCAT, you need to memorize this structure, as well as those of D-glucose, D-mannose, and D-fructose.
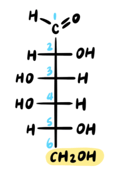
Note that we will not be covering the “long way” of converting a Fischer projection to a Haworth projection. For more on that topic, follow this link!
In our “shortcut” method, we will start with the skeleton of a pyranose ring and number the carbons as follows:
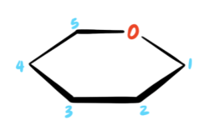
Note that the accepted convention is to place the oxygen atom in the back right position.
Now we must account for all of the carbon atoms. A pyranose ring contains only five carbon atoms, but D-galactose has six carbon atoms! Where do we put the sixth carbon? The sixth carbon (C6) in D-galactose is a CH2OH group, and it is connected to the C5 position. Therefore, we draw a CH2OH group connected to the C5 position in the pyranose ring. The “D” indicates that the CH2OH group must be in the UP position in the pyranose ring.
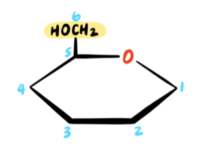
That takes care of the configuration at C5. Now let’s focus on the remaining positions. The configuration at the anomeric carbon (C1) is conveyed in the name of the pyranose ring form. The term alpha means that the OH group at C1 must be DOWN. A mnemonic that I use is: fish (alpha) swim in the sea (down), and birds (beta) fly in the sky (up).
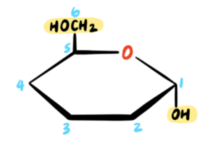
The remaining chiral centers (C2, C3, C4) are drawn using the following rule: any OH groups on the RIGHT side of the Fischer projection will be pointing DOWN in the Haworth projection, while any OH groups on the LEFT will be pointing UP.
Now that we have our Haworth projection, we will draw the chair formation
First, draw the skeleton of a chair and number the carbons as follows:
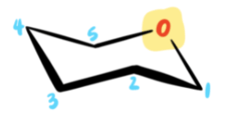
Note how the oxygen is placed on the upper, rear-right corner.
The next step is to draw all substituents on the chair. Each substituent is labeled UP or DOWN and then placed in the appropriate position on the chair conformation:
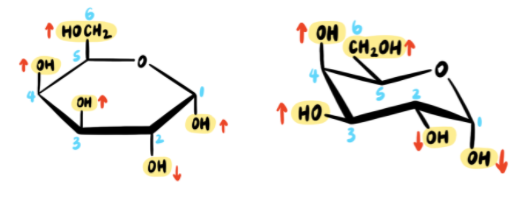
And there we have it!
For practice, try the following problems:
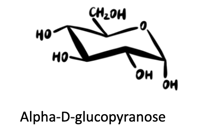
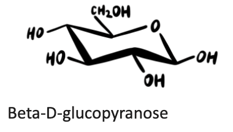
Draw the chair conformation for each of the following compounds:
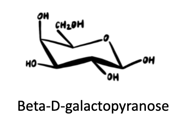
(a) β-d-Galactopyranose
(b) α-d-Glucopyranose
(c) β-d-Glucopyranose
To summarize:
- Draw the skeleton of a Haworth projection, with the ring oxygen in the back right corner. Number each position
- For D sugars, place the CH2OH group pointing up at C5
- Place the OH group at the anomeric position. Alpha = DOWN, and beta = UP
- Place the remaining OH groups at C2, C3, and C4. RIGHT = DOWN, LEFT = UP
- Draw the skeleton of a chair conformation, with the oxygen atom in the upper, rear-right corner.
- Label each substituent as either “UP” or “DOWN” and place each substituent on the chair accordingly.
For more practice, I recommend reading Organic Chemistry by David Klein and the companion series Organic Chemistry as a Second Language.
Answers to practice problems:
Comments