Quickly recognizing the structures of amino acids is an essential, but oftentimes challenging, aspect of both biochemistry courses and standardized tests like the MCAT. Many posts at Cambridge Coaching have provided excellent tips and mnemonics to memorize these based on their names. We’ll take a different approach here leveraging pattern-recognition to help us with some of the amino acids, a great approach for visual-learners, to help you feel confident on test day!
The Basics
Before we dive in, we’ll start with our foundation. The structure of an amino acid consists of 4 main groups:
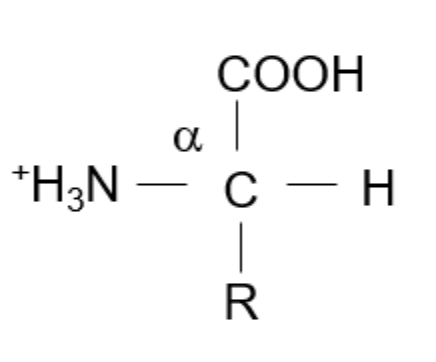
Amino acids are built around a “core” carbon, known as an alpha carbon. They are then surrounded by a Hydrogen, an Amino Group (NH3+), and a Carboxyl Group (COOH). The “R”-group is the only variable region and will determine which specific amino acid we have. In the following sections, focus on how the R-group changes. We will memorize these based solely on structural patterns first – note that these groupings are not based on their characteristics of charge, aromaticity, or polarity.
With this method, those characteristics are best memorized separately through mnemonics. For example, the aromatic amino acids are “FWY” / FreeWay: F for Phenylalanine, W for Tryptophan, and Y for Tyrosine.
Group #1: Built-Up
For ease of visualization, we will cover up the constant groups with a yellow box and focus on the changing R-groups. The first group of amino acids we will explore are Glycine, Alanine, Phenylalanine, and Tyrosine.
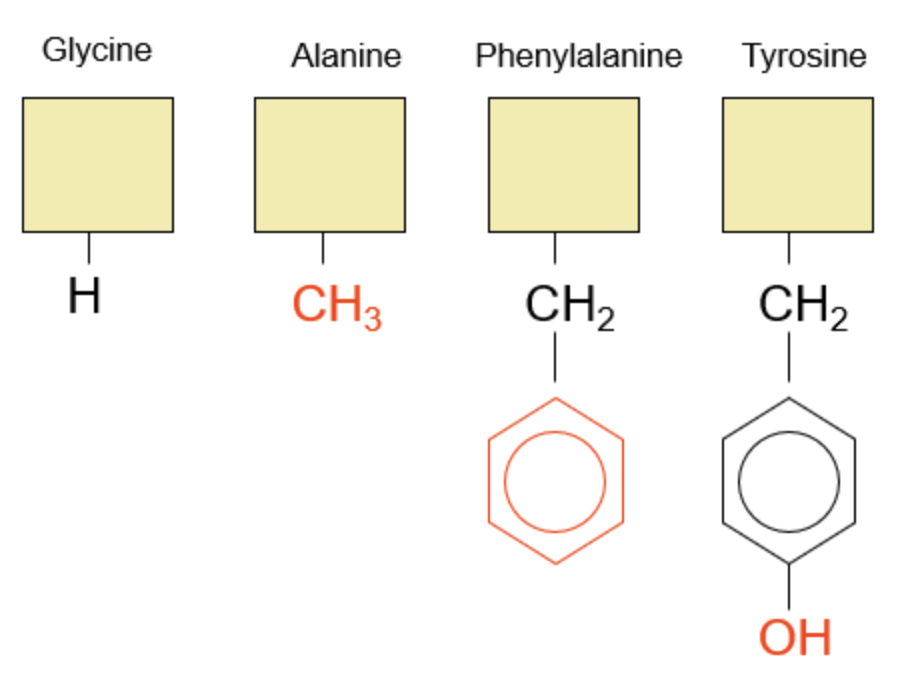
I like to think of these amino acids as an R-group being built up. We first start with our simplest amino acid Glycine, with just a Hydrogen in its R-group. Next, this H grows into a methyl group, or CH3, to form Alanine. A phenyl ring is added to then create Phenylalanine. Finally, we add an alcohol to that ring (phenol) to ultimately make Tyrosine.
Group #2: Acids and Amides
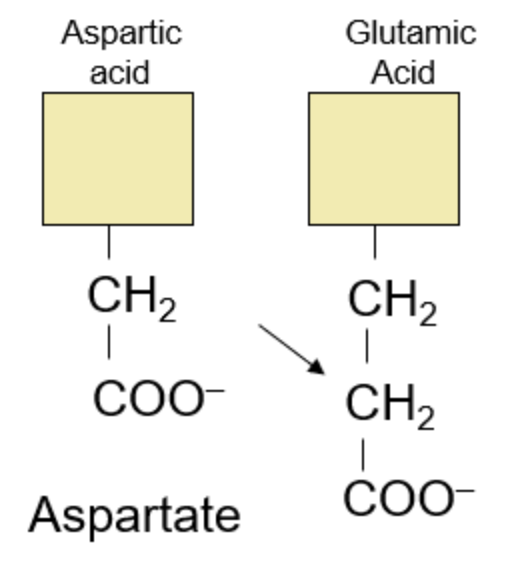
This group focuses on four amino acids and draws patterns in their parallels: Aspartic Acid, Glutamic Acid, Asparagine, Glutamine.
Let’s focus on the acids first:
We start with our most basic, Aspartic Acid. Adding another CH2 group, to simply extend Aspartic Acid, will give us Glutamic Acid. In the alphabet, A comes before G, so I like to view “A”spartic Acid as the shorter one and “G”lutamic Acid as the longer amino acid that comes after.
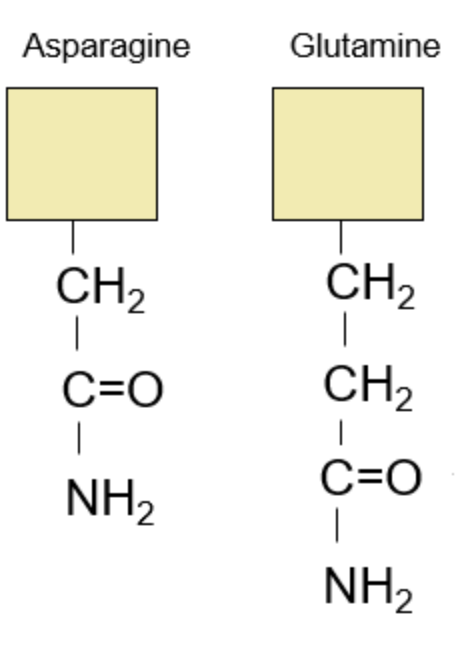
Let’s turn to the amides:
Anytime you see names with words such as -amide or -amine, you can expect to see some form of a nitrogen group in their structure. Therefore, Asparagine is the “nitrogen form” of Aspartic Acid, while Glutamine is the “nitrogen form” of Glutamic Acid. Instead of a carboxyl group as seen in the acids (O=C-O), we replace it with an amide group (O=C-NH2). After that, the same pattern of adding another CH2 group to extend Asparagine into Glutamine applies here!
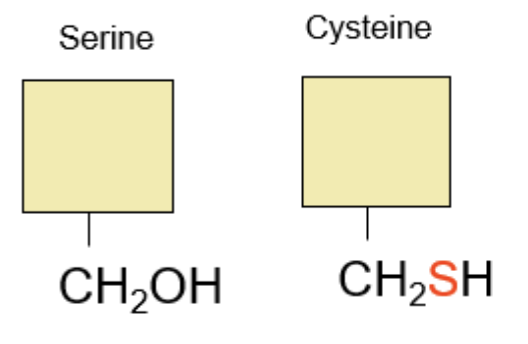
Group #3: Serine and its Cousins
Serine turns into Cysteine when Serine’s Oxygen is replaced by Sulfur.
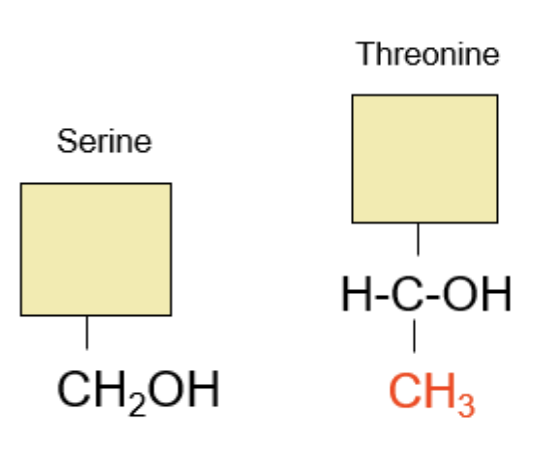
Serine turns into Threonine, when one of its central hydrogens (H attached to Carbon) turns into a methyl group CH3.
Group #4: Lots of Carbons
This group will focus on Valine, Leucine, and Isoleucine, which all have non-polar R-groups filled with hydrocarbons.
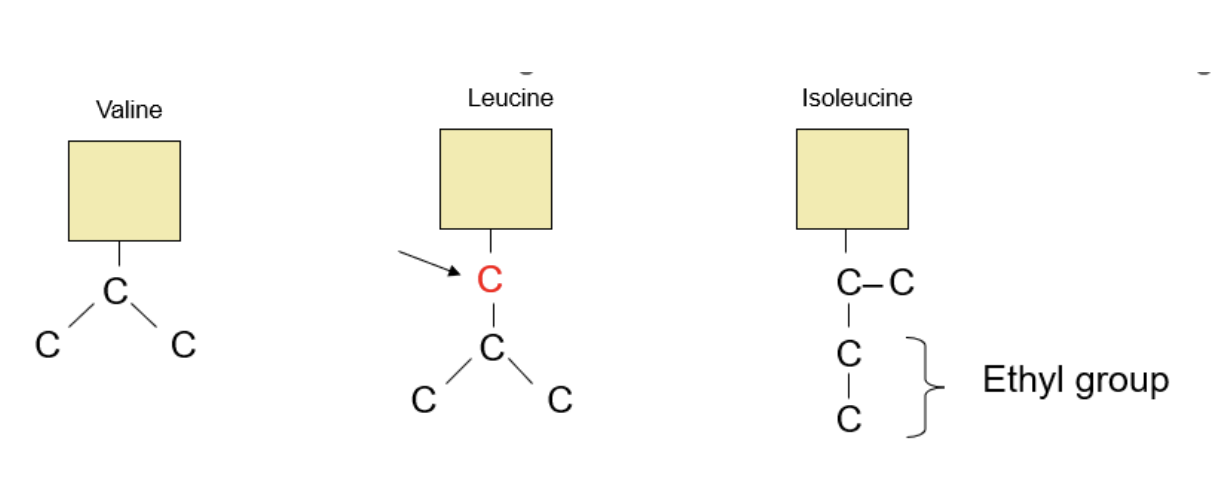
We start with Valine, which like its name, has carbons in a “V”-shape. Adding a carbon creates Leucine, or a “Y” shape.
Isoleucine, with its prefix of “Iso” or “Isomer”, tells us that it will have the same elements as Leucine but just arranged differently. Isoleucine has an “L”-shaped chain of carbons in its R-group. I like to think of Isoleucine as an “Imposter Leucine”, as an “L”-shaped chain would have been better suited for Leucine!
Group #5: The Others
These amino acids unfortunately don’t have any patterns, so these structures need more rote repetition to memorize. However, at least for tests like the MCAT, amino acids are tested by *recognition* and not *recall*. You won’t have to draw out these structures on test day. Thus, you can recognize these based on how different their structures are to each other, highlighted in teal.
Positively-Charged Amino Acids:
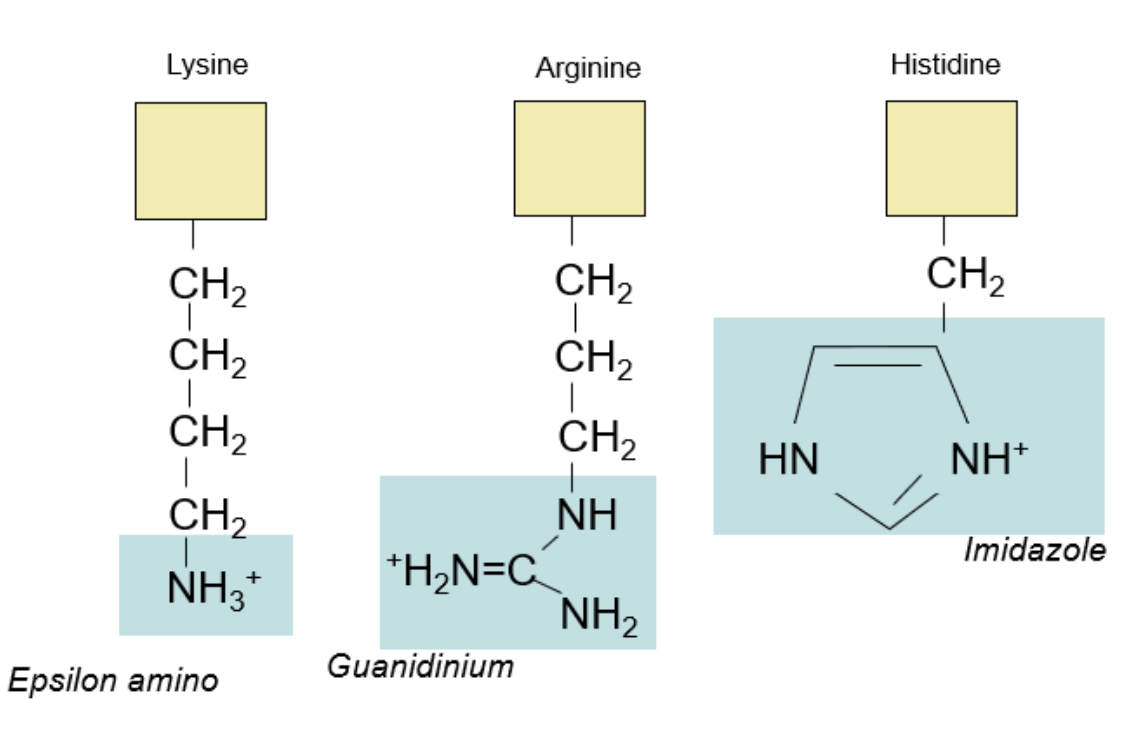
Other Amino Acids
Tryptophan is unique for its double-ringed structure, one 5-carbon and another 6-carbon attachment.

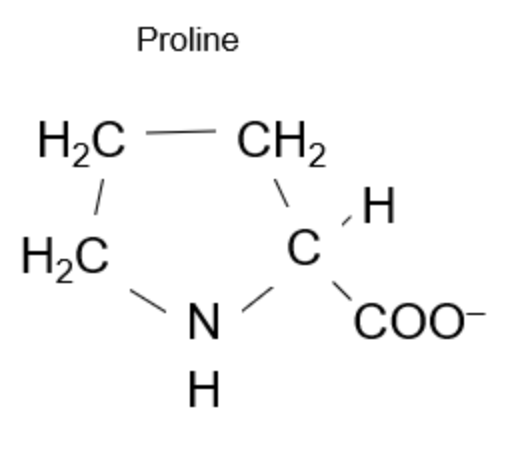
Proline is unique for the way it makes a “Pentagon” and looPs back on itself.
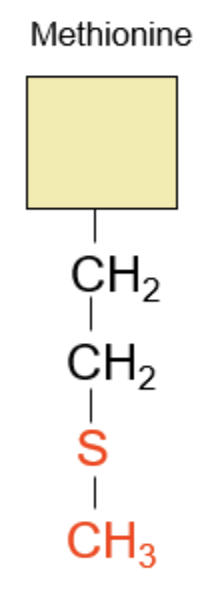
Lastly, methionine. The “thio” prefix/suffix tells us there will be a sulfur.
Comments