We all know Orgo 1 professors love stereoisomers. Consider the equation A + B = C. Most professors expect you to fill in the question mark with all possible products and then indicate the major product(s), while other professors may provide you a potential C and then ask you if the statement is True or False.
Whatever the case, you've likely learned alkene addition reactions first, and you’re likely trying to memorize the three hallmark hydration reactions:
acid-catalyzed hydration: H2SO4 (aq)
oxymercuration-reduction: Hg(OAc)2 (aq) followed by NaBH4
and hydroboration-oxidation: BH3-THF followed by H2O2, NaOH (aq)
Yes, it’s important to memorize that:
acid-catalyzed hydration results in syn + anti addition
oxymercuration-reduction results in anti addition
and hydroboration-oxidation results in syn addition
However, it’s about here where the memorization should stop. It’s tempting to memorize that acid-catalyzed hydration results in racemic mixtures, but this simply isn’t always the case. Think about it. If you’re powering through this mechanism and there’s already a chiral center present elsewhere, you’re going to be left with two diastereomers, not two enantiomers, and therefore not a racemic mixture. For example:
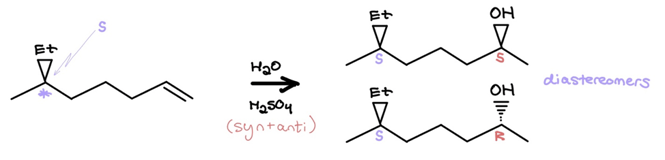
The latter two hydration reactions have a different issue. Namely, it’s impossible to mentally envision their products without showing any work.
What am I suggesting? Mechanism. Only by learning to power through mechanisms quickly and with confidence will you be able to determine and compare alkene hydration products. The following methods are how I teach my students to systematize the process.
Acid-Catalyzed Hydration (syn + anti addition)
Since this mechanism is carbocation-based, we expect our H2O nucleophile to attack the planar sp2 carbocation from the top and bottom face with equal probability, which results in equal syn + anti addition – right?
But wait! We already forgot something. The mechanism is carbocation-based . . . meaning rearrangements like hydride and alkyl shifts are possible, and things can get messy.
With this in mind, the system for determining all products of this hydration method is as follows:
- Create carbocation — listen to Markovnikov’s rule
- Look for a potential better carbocation (i.e. hydride/alkyl shifts)
- Account for equal syn + anti addition at all carbocations
- Addition at original carbocation gives you minor products
- Addition at better carbocation gives you major products
Example:
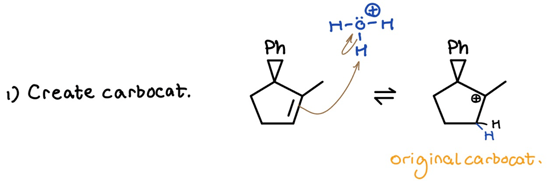
I put the original carbocation in a place Markovnikov would respect. Just note that your professor may want you to show anti-Mark. products to technically satisfy the all products condition. Moving on . . .
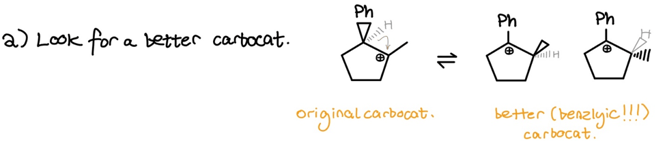
I performed a hydride shift to get to a benzylic carbocation–the best carbocation we got! As you can see, we technically get two new carbocations since the hydride can shift to either face of the original carbocation. To finish up . . .
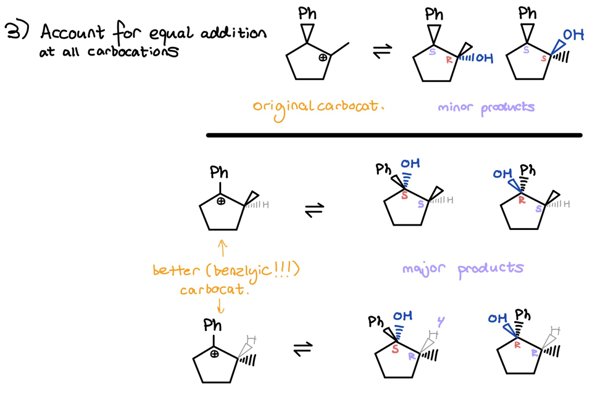
There’s no way we could’ve done that in our heads!
Oxymercuration-Reduction (anti addition)
Ah . . . Three-membered intermediates. Tackling these is best shown with an example.
Example:
Following Hg(OAc)2 dissociation, the nucleophilic pi-bond of the below alkene can attack the resultant Hg(OAc)+ from either the top or bottom face. I always show attack from the top face to stay consistent when solving different problems, and I recommend you do the same. Check it out . . .
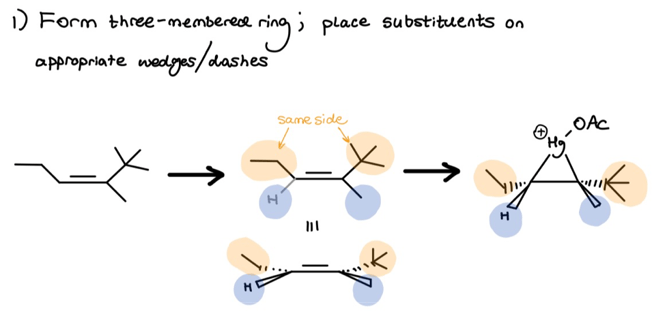
I labeled the above substituents in orange and blue to emphasize that, during ring creation, you must preserve the original stereochemistry. I rotated the middle molecule to better show this stereochem.
Next, you should recognize that three-membered ring opening is an anti process. We now draw an “anti-skeleton” based on where the nucleophile is going–in this case, Markovnikov placement.
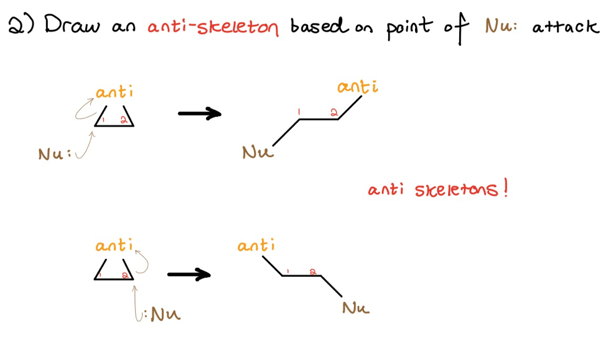
Again, which anti-skeleton you choose—top or bottom—depends on where the nucleophile attacks.
We next fill in and populate the anti-skeleton. Keep substituents previously on wedges on wedges, and keep substituents previously on dashes on dashes. In other words, don’t touch anything!
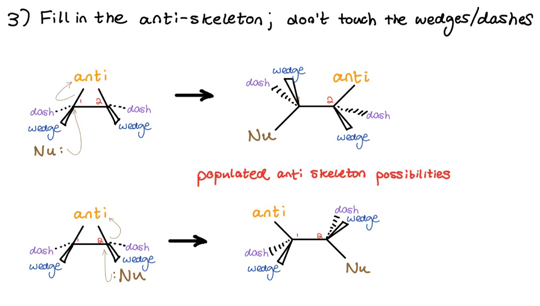
Using our beginning example and skipping the mechanistic details, the process looks like this:
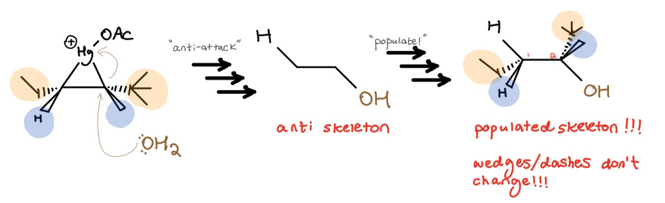
In summary:
- Form a three-membered ring on one face only, and place alkene substituents on appropriate wedges/dashes
- Draw an anti-skeleton based on point of Nu: attack
- Populate the anti-skeleton; don’t touch the wedges/dashes
Hydroboration-Oxidation (syn addition)
Shortcutting a hydroboration-oxidation mechanism is similar to powering through an oxymercuration-reduction mechanism. The main difference is we use two “syn-skeletons.” Using the same example with the new conditions:
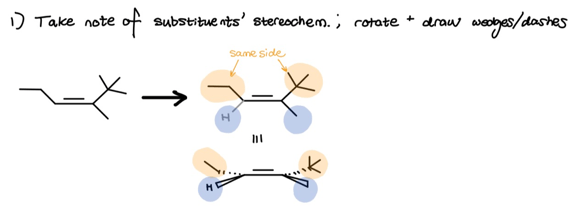
As you can see, we once again took note of what substituents are on what side of the double-bond. Since hydroboration-oxidation follows a syn-mechanism, we must now draw two syn-skeletons to account for both top and bottom face addition.
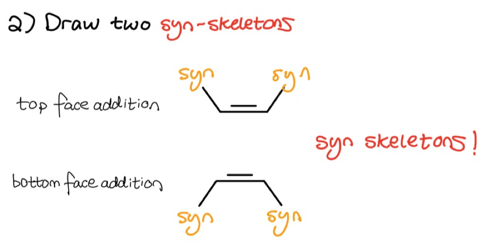
Finally, we populate both skeletons and keep in mind that the hydroxyl goes to the less substituted (anti-Mark.) side. Wrap up by filling in the substituents. Don’t touch the wedges or dashes.
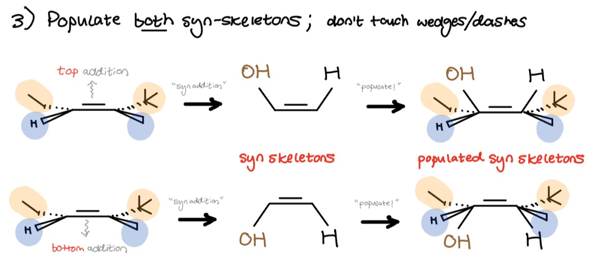
In summary:
- Take note of substituents’ stereochem; rotate alkene to show wedges/dashes
- Draw two syn-skeletons
- Populate both syn-skeletons; don’t touch the wedges/dashes
Conclusion
Phew! You can now systematically power through the three main alkene hydration mechanisms. My strong advice is to get comfortable determining the resultant stereochemistry. On exams, professors love to provide you with potential products and ask if they are, in fact, possible products. The only way to safely solve this problem with confidence is to compare the configurations (R/S) of your own products with the configurations of those given by the professor. Have fun!

Comments