I’ve already covered how to easily manage carboxylic acid derivative formation and manipulation using the Reactivity Hill.
Say we’re tired of whatever derivative we just created and want to bring the derivative back to its parent acid (the particular acid the derivative came from). There are two ways to “take home” any acid-derivative. We can account for these “take home” conditions in the Reactivity Hill scheme we’ve already seen.
“Take Home” Condition #1: Acid Hydrolysis (H2O, HCl, Heat)
The first “take home” condition combines water, acid, and heat. As we know, water isn’t the greatest nucleophile, so hydrolysis of less reactive derivatives like amides requires a catalytic amount of strong acid (like HCl) in addition to heat to favor the “take home” hydrolysis. (The strong acid protonates the derivative’s carbonyl oxygen, thus making the carbonyl carbon more electrophilic for water.)
On the other hand, extremely reactive derivatives like the acid chloride don’t need this acid catalyst for an efficient hydrolysis.
Where do we draw the line for when we need or don’t need to include acid and heat? This may depend on your professor, but it’s usually safe to throw in both if you’re not sure.
“Take Home” Condition #2: Basic Saponification (H2O, NaOH, Heat)
The second way to get a derivative home is a 2-step process. The first step is basic saponification, which involves hydroxide attack of the derivative’s carbonyl carbon. Following this hydrolysis mechanism, we’re left with a carboxylate because we’re trying to create an acid in a basic system. In other words, even if we make the acid, there’s lots of NaOH around to deprotonate it. I refer to the carboxylate as “the doorstep” of the ongoing “take home” analogy.
How do we enter the “home” from “the doorstep”? (How do we get to the parent acid from the carboxylate?)
Simply use some strong acid in water. (Think about why we must use a strong acid…)
Incorporating “Take Home” Conditions into the Reactivity Hill
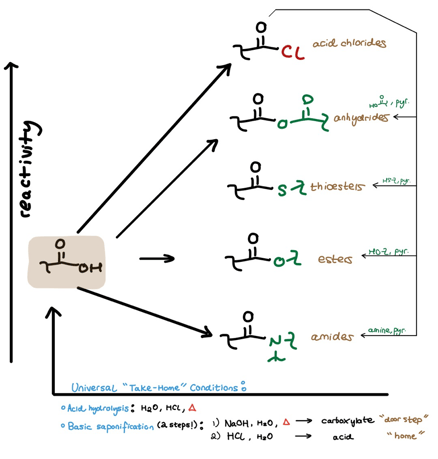
The “take home” conditions are accounted for in the bottom-most arrow going from right to left. We can now see the cyclical nature of the Reactivity Hill:
We can go Left to Right (direct derivatization of an acid)
We can go Top to Bottom (“moving downhill” from an acid chloride or other higher derivative)
We can go Right to Left (“taking home” any derivative back to the parent acid)
Conclusion
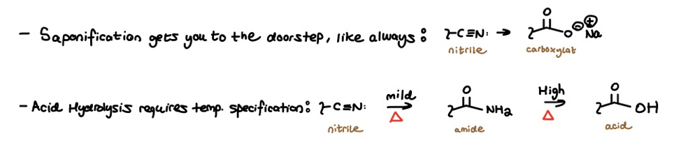
The Reactivity Hill is complete… But wait, there’s more. What about the silent acid-derivative: the nitrile? The nitrile doesn’t fit neatly into the Hill because it doesn’t have a carbonyl. However, as an acid-derivative, the nitrile does obey “take home” conditions with a small twist.
Acid hydrolysis of nitriles results in an amide or acid depending on the implemented temperature: mild or high.
Meanwhile, basic saponification gets nitriles to “the doorstep” as predicted. Therefore, don’t forget to follow up nitrile saponification with HCl (aq) if you want to get all the way home to the parent acid.

Comments