A common exam question that comes up in both Orgo 1 and Orgo 2 requires students to determine a reaction’s equilibrium position. The question usually looks something like this:
Are the reactants (Keq < 1), or are the products (Keq > 1) favored in each of the below reactions:
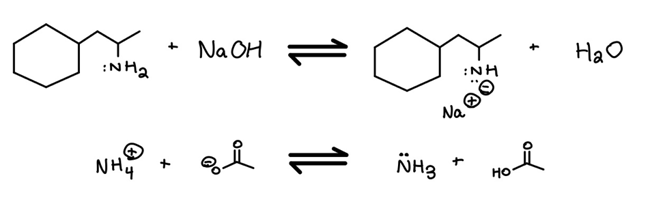
Solving this type of question is easy with a little help from the Protocol and one more idea.
Applying the Protocol
We all know that nature hates energy–the lower the energy, the better. With this idea in mind, don’t forget we can use the Protocol to evaluate the relative stability of two negative charges, where the more stable negative charge is lower in energy.
Thus, one way to determine whether the reactants or products are favored in an equilibrium is to compare the stabilities of two negative charges on opposite sides of the equilibrium-arrows. Whichever side has the more stable negative charge is favored because this side is lower in energy.
One way to think about why this statement is true is to consider what happens once you get to the more stable negative charge. If you just got to a more stable position, you’re less likely to react in the backward direction because you’re happy where you are–a place of low energy. In other words: once you get there, you stay there!
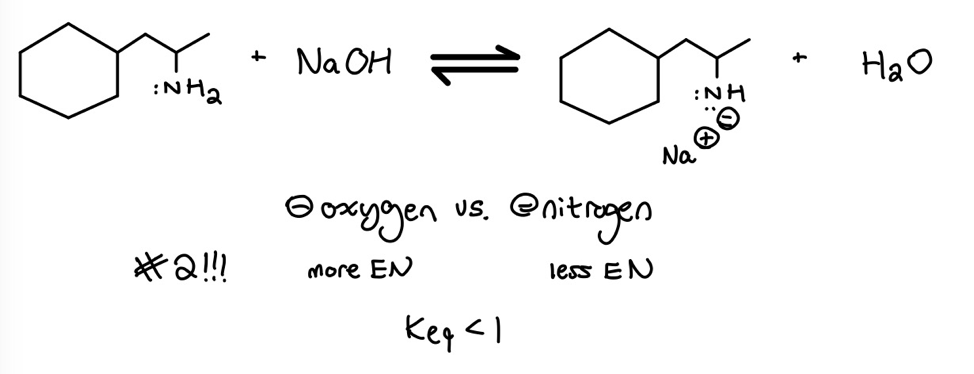
In the above example, we’re putting the protocol to work by comparing a negative charge on an oxygen to a negative charge on a nitrogen. Since we’re comparing the charges on two atoms in the same row of the periodic table, we use electronegativity (#2 on the Protocol) to determine the relative stabilities.
A Notable Exception:
It’s worth noting that Orgo 1 introduces a major exception to the Protocol. Namely, Orgo 1 says you can use sodium amide (NaNH2) to deprotonate terminal alkynes. (Remember, this step is important for Orgo 1’s only path toward C-C bond formation.) I know, I know: too many words. Here’s a visual:
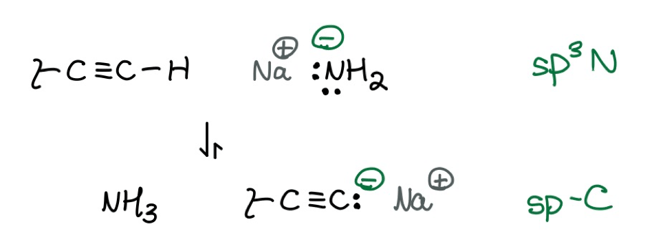
What you should notice is that the equilibrium favors the negative charge on the carbon instead of the nitrogen. This contradicts the Protocol’s prediction that a negative nitrogen beats a negative carbon. However, once you notice the hybridization of both atoms (pointed out in green, above) you can see that we have two competing stabilizing factors: the electronegativity of the atom and the electronegativity effect of the atoms’ hybridization.
This is an exception to memorize, and it’s easy to remember since we do it all the time to create C-C bonds.
Proton Transfer is #1
What if there aren’t two negative charges on opposite sides of the arrows? A second way to evaluate an equilibrium is to consider that the transfer of a proton from good bronsted acids to good bronsted bases is always favorable.
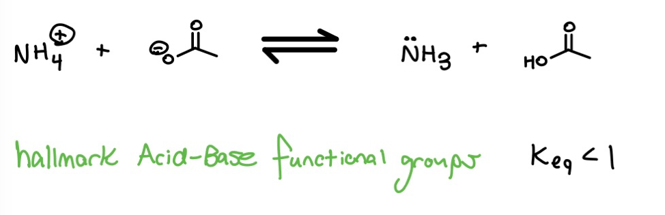
Conclusion
Once you understand the Protocol and the great favorability of proton-transfer, determining the equilibrium position of reactions is straightforward. If you come across equilibrium questions on your exam, don’t forget to ask yourself if you can compare two negative charges on opposite sides of the arrows. (And don’t forget to double-check you’re not looking at our notable exception.)
If there aren’t two charges to compare, then consider that you may be looking at a proton-transfer between common orgo acids and bases (e.g. amines, strong acids, carboxylic acids).
Best of luck!

Comments