
So your professor says your Orgo 1 final will have a few synthesis problems. The good news: you’ve only learned a handful of reactions. Namely, you’ve learned how to manipulate alkenes and alkynes, and you know a little about radicals, substitution versus elimination, and the chemistry of alcohols, thiols, ethers, and epoxides. The bad news: well–there’s none to give. Managing synthesis problems in Orgo 1 is easy when you learn to look for red flags!
Red Flags
All my students know I summon the term red flag when dealing with synthesis.
A red flag is a transformation that we’ve mutually agreed is accomplishable in only one way.
Looking for red flag transformations is like filling in a crossword puzzle’s easy words first so that the puzzle’s harder words become more obvious. Put into practice, imagine your professor asks you to transform molecule A into molecule F in as few steps as possible, and he or she doesn’t give you any hints. The problem looks something like this:
A to __ to __ to __ to __ to __ to __ to __ F.
Number of steps unknown.
Now, imagine you’ve spotted one of our red flags. The problem now becomes this:
A to __ to __ to D to E to__ to __ to F.
Number of steps now more obvious.
By spotting a single red flag transformation (D to E), you’ve both discovered two intermediates and made it your new job to get from A to D and from E to F. You’ve also probably gained a better idea of how many steps it’ll take to do the original A to F transformation. For example, you may see that you can actually get from A to D in just one step, and the problem now looks like this:
A to D to E to __ to __ to F.
Whatever the case, use the following two red flags to guide your synthesis.
Red Flag 1: Carbon-Carbon Bond Count Increase
There’s typically only one way to create carbon-carbon (c-c) bonds in Orgo 1. It’s accomplished by deprotonating a terminal alkyne and throwing the resultant nucleophilic conjugate base at some alkyl halide. Remember, this follows an SN2-type mechanism because we’re using a good nucleophile with a full-blown negative charge to attack our electrophile, the alkyl halide. In other words, don’t forget a chiral carbon’s configuration will be inverted, and don’t forget the alkyl halide must be a methyl or 1o alkyl halide to avoid steric prohibition of the mechanism.
Therefore, if you spot an increase in the c-c bond count, you now know you must at some point:
- Create a terminal alkyne (to be deprotonated and made into a nucleophile)
- Create a methyl or 1o alkyl halide (to be attacked by the above nucleophile)
Example:
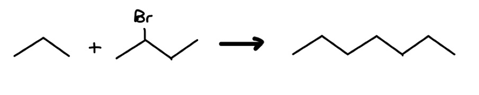
Clearly, there’s an increase in the c-c bond count: we’re looking at red flag 1. Let’s label our carbons and draw a potential point of connection.
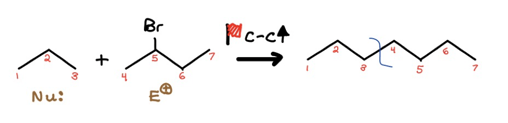
Alright, you may’ve noticed I also arbitrarily labeled our potential nucleophile and electrophile. That was me picking my poison; your own poison could’ve been different than mine. Just keep in mind one path may be shorter and reap you more exam points. Moving on, let’s fill in the red flag transformation.
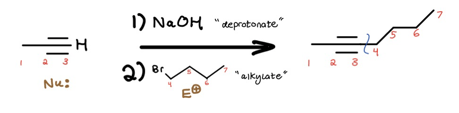
Voilà! We’ve increased the c-c bond count. To finish the crossword puzzle, your new jobs are as follows:
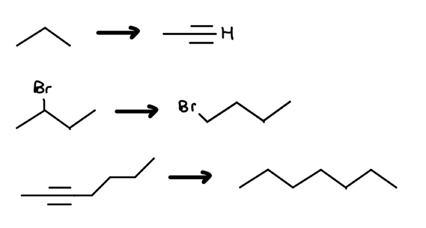
Red Flag 2: Carbon-Carbon Bond Count Decrease
Ozonolysis is the only way to decrease the c-c bond count in Orgo 1. Thus, if you ever see a product with less carbons than the original molecule, you’ve spotted red flag 2.
If you spot a decrease in the c-c bond count, you now know you must at some point:
- Create an alkene (to be cleaved by ozonolysis)
or - Create an alkyne (to be cleaved by ozonolysis)
Side Note: Remember, we typically use a reductive workup (dimethyl sulfide, “DMS”) for alkene ozonolysis since it leaves us with ketones and aldehydes only. We don’t like oxidative workups (hydrogen peroxide) because it may leave us with a carboxylic acid, which we can’t really transform until Orgo 2.
Example:
Since the reactant is fragmented into two products, we’re clearly looking at a c-c decrease and red flag 2. Let’s label our carbons and draw in the point of disconnection.
Great! Now we can fill in the leftover gaps: synthesize the above alkene from the original molecule, transform 2-methylpropanal into the final alcohol product (2-methyl-1-propanol), and transform acetophenone into the final alkene product (styrene). In other words, your jobs are as follows:
Much more manageable!
Conclusion
Looking for red flag transformations when approaching complex synthesis problems is a great way to avoid the question, “where do I start?” Going forward, label your carbons and keep your eyes open for c-c bond formation and cleavage. If you come across either of these red flags, structure your synthesis around them and fill in the remaining blanks. Your brain will thank you!
Are you looking for an organic chemistry tutor in Boston or New York?
Hunkering for more on the subject? Read on, dear reader, read on!

Comments