
A lot of students get overwhelmed with all the manipulations we can do with carboxylic acids and their derivatives. Outside of being able to deprotonate carboxylic acids if we so desire, we can also directly derivatize them. For example, we can transform them into acid chlorides. Acid anhydrides. Esters. Amides. We can also transform acid chlorides into every other derivative, anhydrides into every other derivative but the acid chloride, thioesters into every other derivative but the acid chloride and anhydride…
You get the point–this is a lot to keep track of. Let’s simplify what we can do with acids and their derivatives utilizing what my former professor calls the “Reactivity Hill.”
The Reactivity Hill: Left to Right
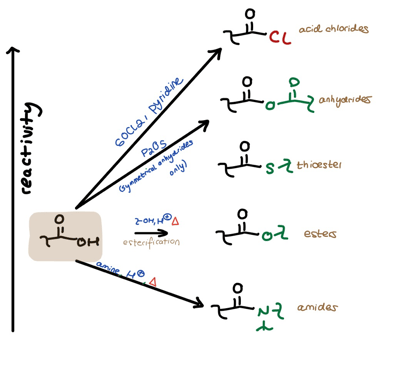
Let’s break down the reactivity hill. First, let’s move from left to right. To the left we have the regular ol’ carboxylic acid highlighted in brown. To the right we have all its derivatives, stacked vertically in order of relative reactivity. The arrows going from the acid (left) to the derivatives (right) show the several ways we can directly derivatize the carboxylic acid using different condition sets.
Left to Right:
-We can use thionyl chloride and pyridine to get to acid chlorides,
-We can use P2O5 to get to symmetrical anhydrides,
-We can use a generic alcohol + acid + heat to get to esters,
-And we can use a generic amine + acid + heat to get to amides.
You should memorize these conditions.
The Reactivity Hill: Top to Bottom (“Moving Downhill”)
Once you’ve memorized the various ways to directly derivatize the acid, you should next understand and memorize the vertical order of the derivatives to the right.
(The key to understanding the relative reactivity of the derivatives is to evaluate the magnitude of each carbonyl carbon’s partial positive charge. The greater the partial positive charge, the more electrophilic that carbon is and the more reactive it is to a wandering nucleophile. Here’s another great place to apply the Protocol. Why is the partial positive of the ester greater than the partial positive of the amide?)
Got the order down? Okay–here comes the beauty of the Reactivity Hill. You can move downhill from any higher position.
This is why lots of professors and textbooks talk about the great usefulness of acid chlorides. From the acid chloride you can create any other acid-derivative using a nucleophile of your choosing along with some pyridine. Look at the arrows to the right and the reagents in green to see this.
Similarly, you can move downhill from any other derivative: just use the appropriate nucleophile. (No pyridine is necessary when you’re not moving down from the acid chloride because you don’t have to worry about soaking up any resultant HCl.)
Conclusion
There are technically over a dozen ways to manipulate the carboxylic acid and its derivatives. Understanding the Reactivity Hill is a way to bypass memorizing every single thing one of these transformations.
From here, you can use the Hill to make other types of predictions. What if both a thioester and ester are present on a single molecule, and you’re told to combine this molecule with a single equivalent of some nucleophile? Will the nucleophile attack the thioester or ester?
So, the Hill is an all-around great way to standardize how you approach acids and their derivatives. I encourage you to keep it in the back of your mind when you encounter these groups!
Do you need help in organic chemistry? Reach out today to connect with one of our chemistry tutor in Boston, New York, or online.
Looking to read more on the subject?

Comments